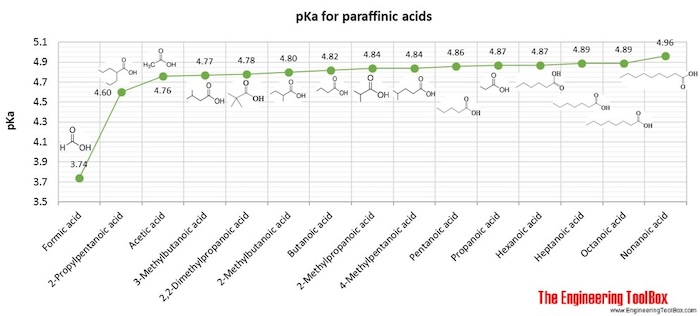
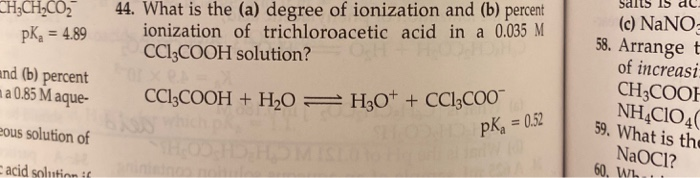A Reliable and Efficient First Principles-Based Method for Predicting pKa Values. 2. Organic Acids | The Journal of Physical Chemistry A

How to determine the strength of facial peels: debunking the myths and to determine how strong different peelings really are - nunii
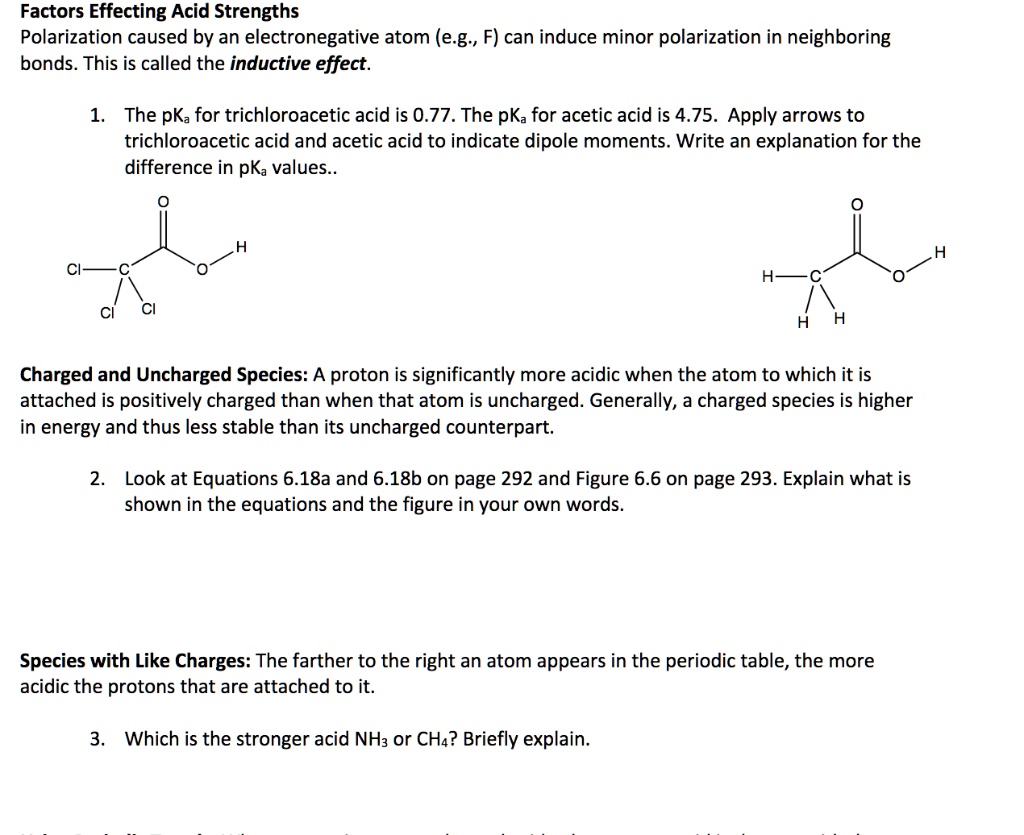
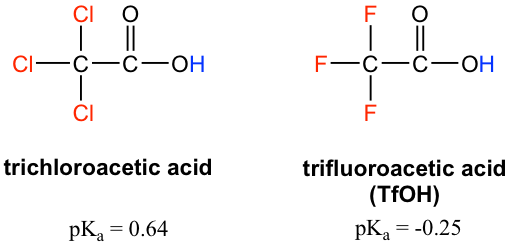
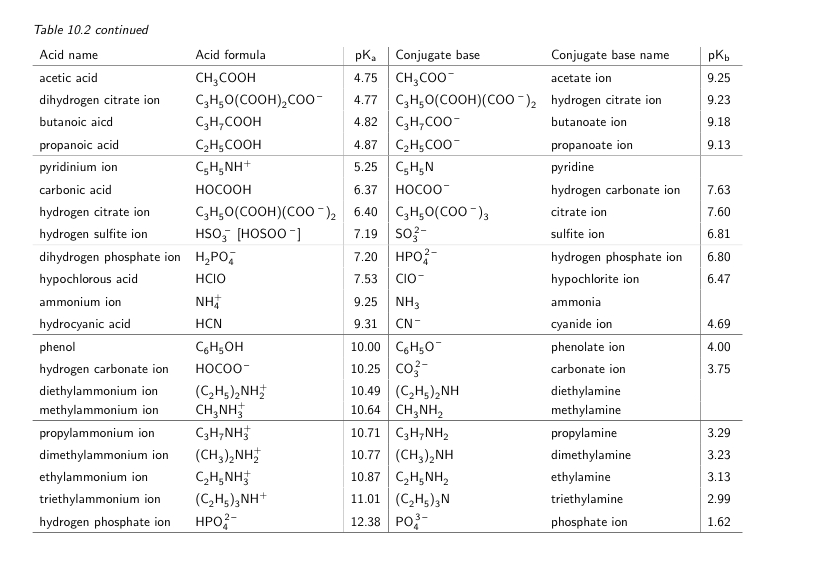
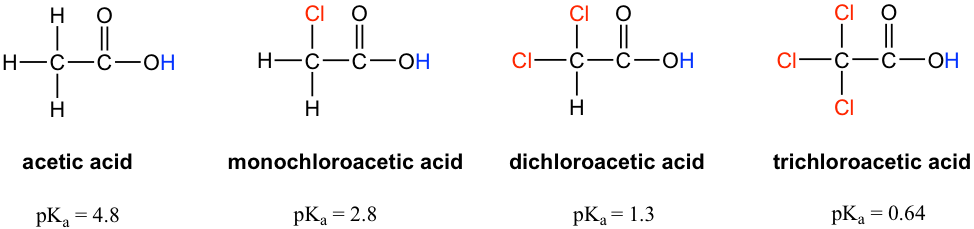
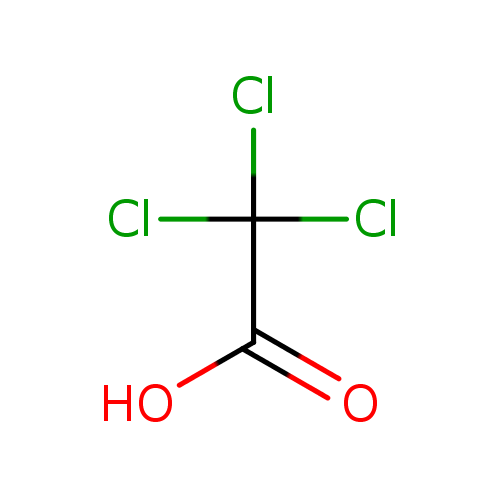
SOLVED: Factors Effecting Acid Strengths Polarization caused by an electronegative atom (e-g , F) can induce minor polarization in neighboring bonds. This is called the inductive effect: The pKa for trichloroacetic acid

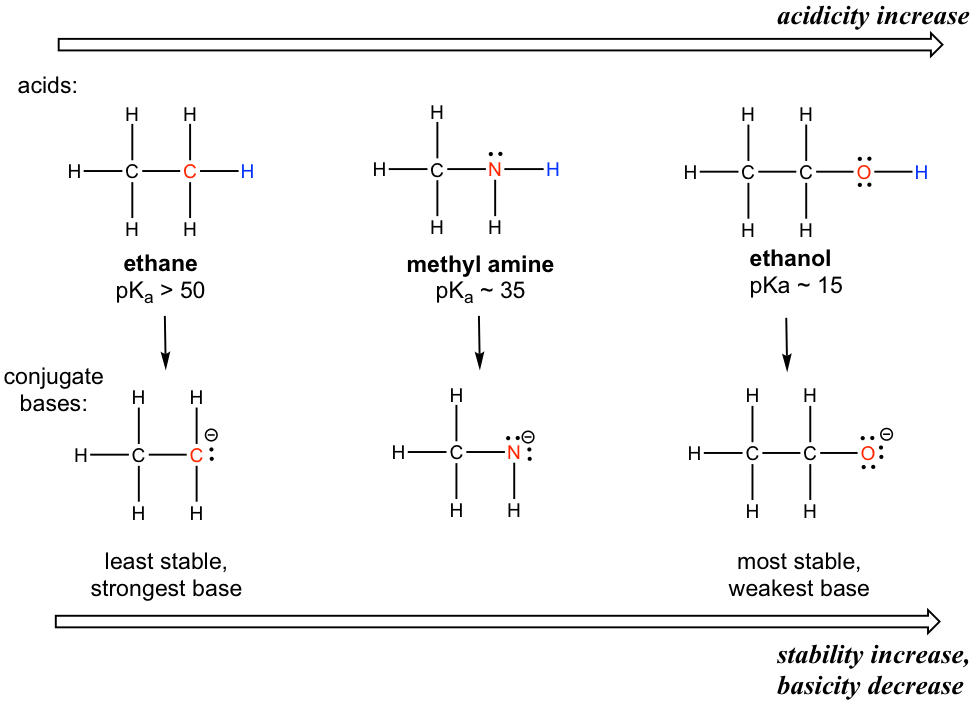
Chloroacetic acid is a stronger acid than acetic acid. Give Reason. | CurlyArrows Chemistry Tutorials

A Reliable and Efficient First Principles-Based Method for Predicting pKa Values. 2. Organic Acids | The Journal of Physical Chemistry A

SOLVED: Warts beware. The pKa of acetic acid is 4.76 and the pKa of trichloroacetic acid, which is used to remove warts, is 0.7. Calculate the dissociation constant of each acid. Which