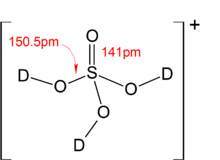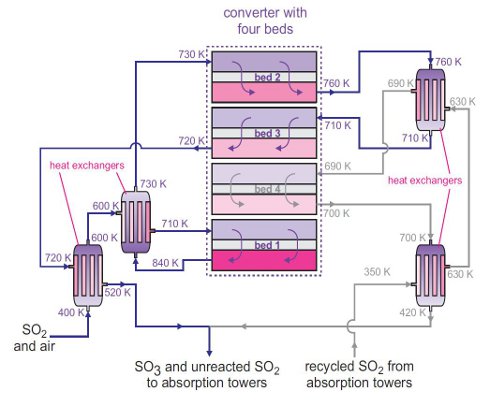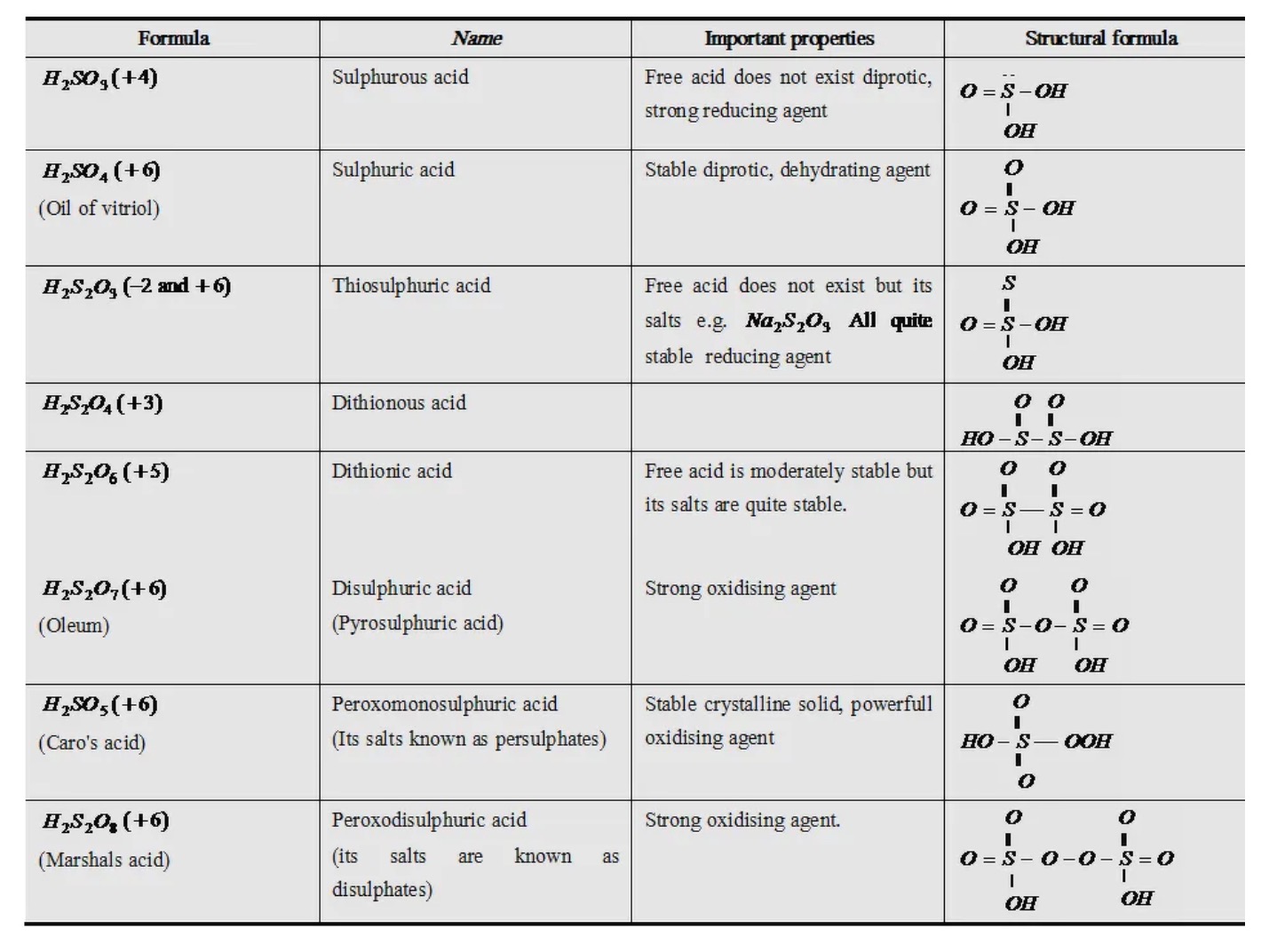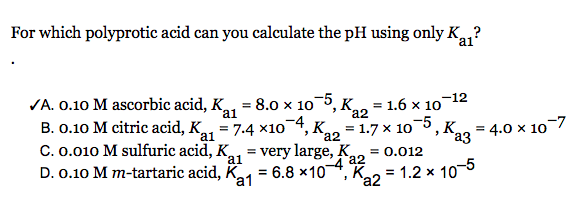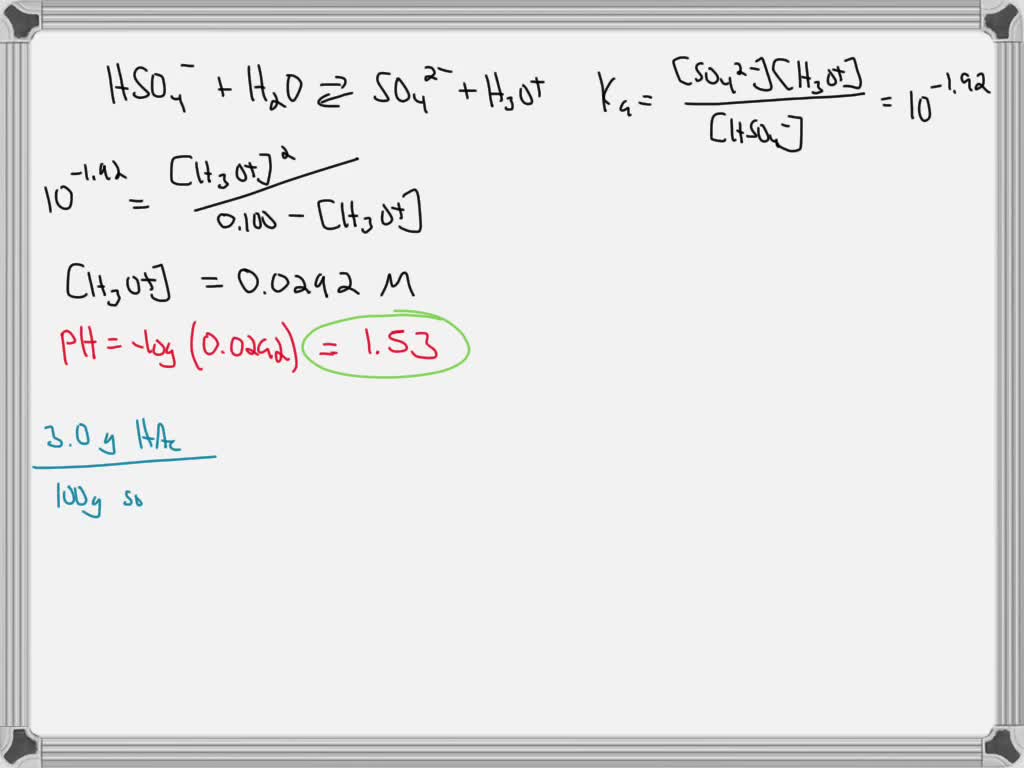
SOLVED: 8) Sulfuric acid is a strong acid, and the pKa 2 of HSO - 4 is 1.92. What is the pH of a 0.100 M NaHSO 4 solution? 9) What is

ACP - New particle formation from sulfuric acid and ammonia: nucleation and growth model based on thermodynamics derived from CLOUD measurements for a wide range of conditions

How much heat does 240 millilitres sulfuric acid make when 240 millilitres of water is added? - Quora

Dissociation Constants of Perchloric and Sulfuric Acids in Aqueous Solution | The Journal of Physical Chemistry B

Ammonia Catalyzed Formation of Sulfuric Acid in Troposphere: The Curious Case of a Base Promoting Acid Rain | The Journal of Physical Chemistry A

Why're hydrochloric acid, nitric acid, and sulfuric acid strong acids, while hydrofluoric acid and acetic acid weak acids?
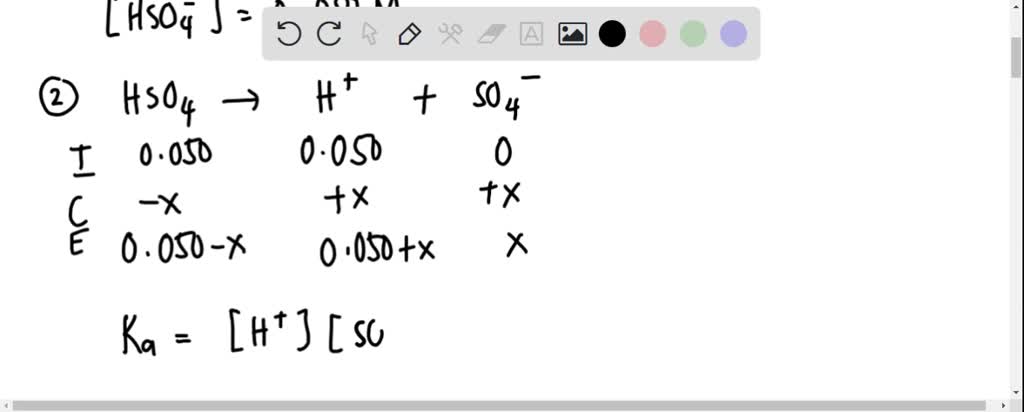
![SOLVED: Example 14.13 What is the pH of a 0.0025 M sulfuric acid solution? What is [S042-]? HzSO4 (aq) + Hz0 () H:O+ (aq) + HSO4 (aq) HSOz (aq) + HzO () SOLVED: Example 14.13 What is the pH of a 0.0025 M sulfuric acid solution? What is [S042-]? HzSO4 (aq) + Hz0 () H:O+ (aq) + HSO4 (aq) HSOz (aq) + HzO ()](https://cdn.numerade.com/ask_images/0c1eac2937604c57b404c3a0e47eb149.jpg)
SOLVED: Example 14.13 What is the pH of a 0.0025 M sulfuric acid solution? What is [S042-]? HzSO4 (aq) + Hz0 () H:O+ (aq) + HSO4 (aq) HSOz (aq) + HzO ()