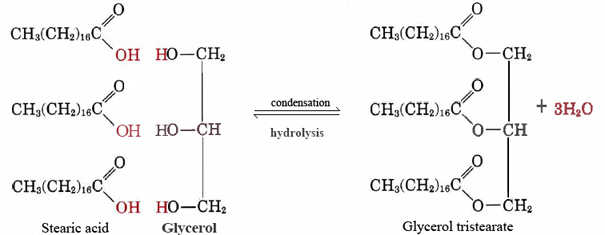
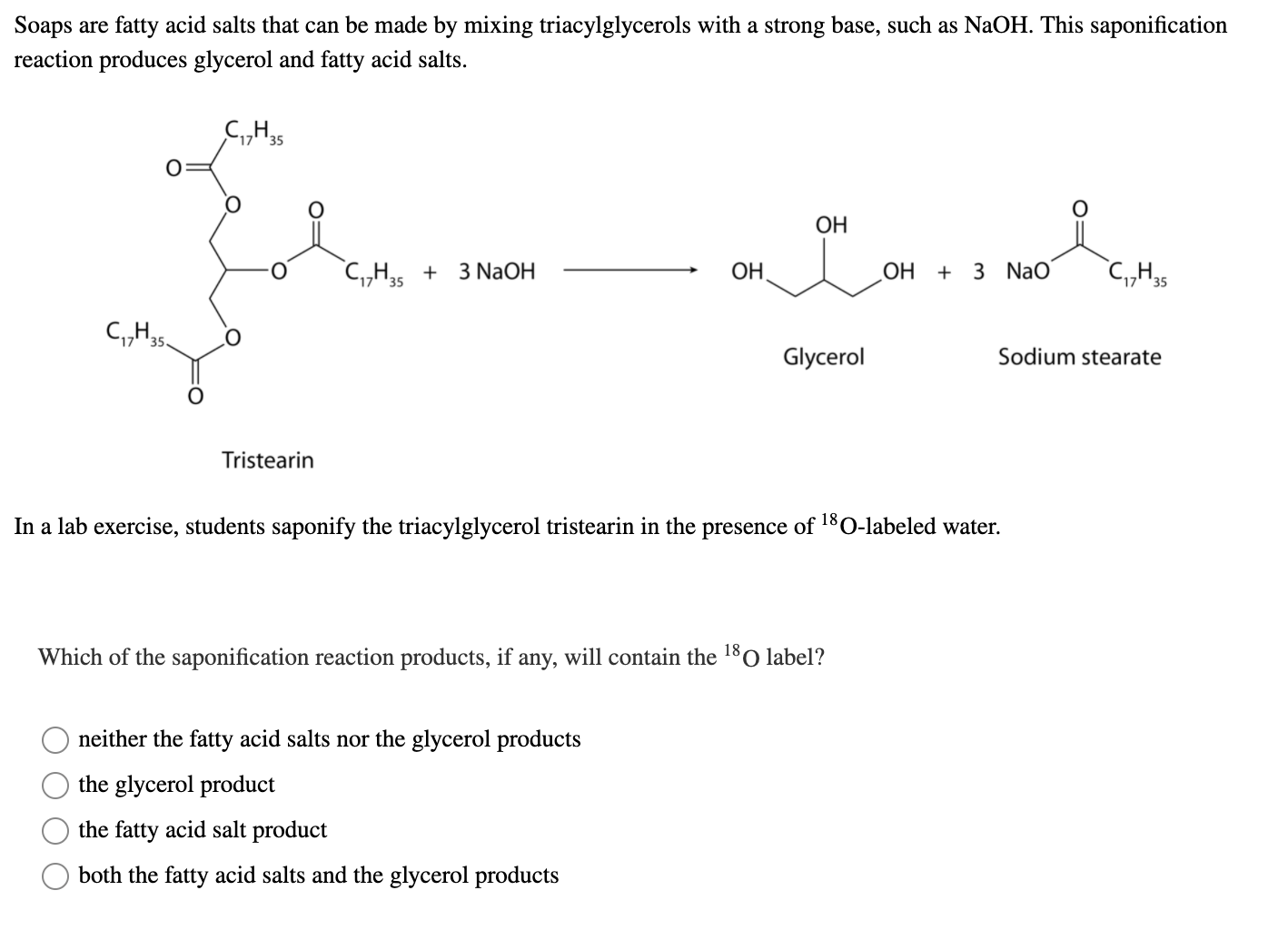
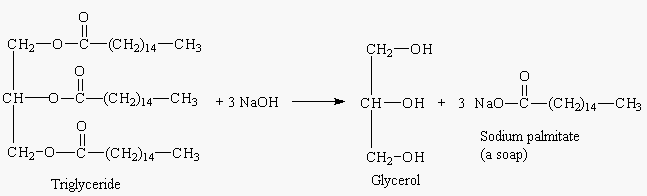
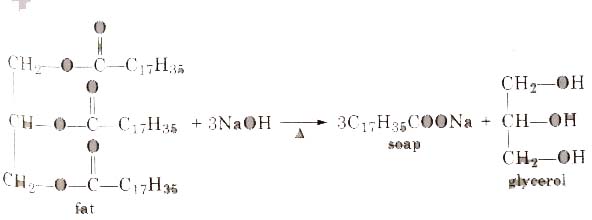SOLVED: Calculate the theoretical yield of sodium stearate (soap). 500 g triglyceride of stearic acid. We have an atom economy of 90.9%. The process is triglyceride of stearic acid + 3 Sodium

SOLVED: In the last step; we attempt to dissolve small amount of stearic acid in a few milliliters of 0.1 M NaOH: How would the solubility of the stearic acid change in
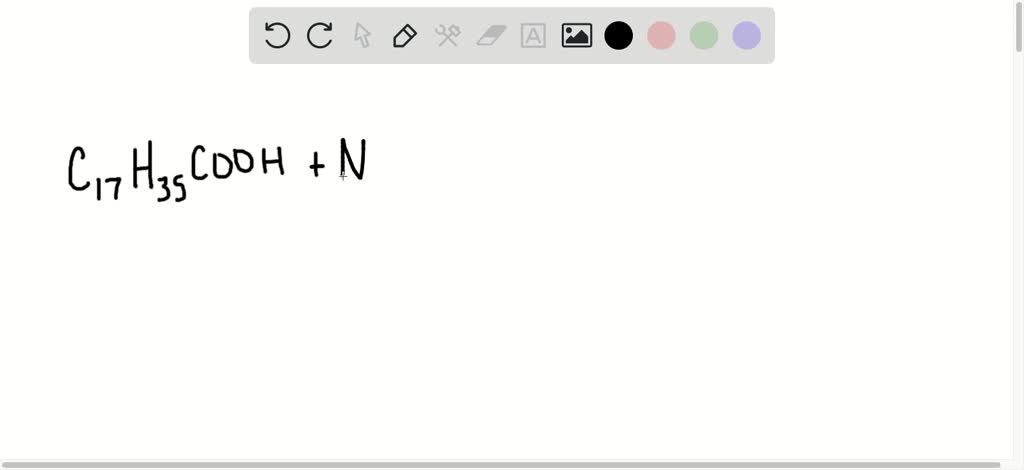
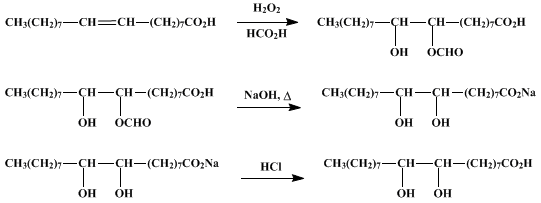
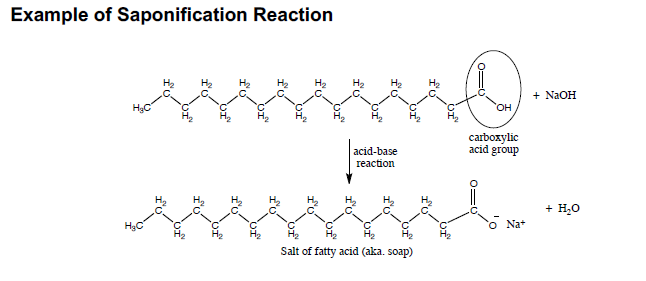
SOLVED:Write the equation representing the formation of a soap molecule from stearic acid, C17 H35 COOH, and sodium hydroxide.

OneClass: Write a chemical reaction between stearic acid CH3(CH2)16COOH and sodium hydroxide to form ...
Experimental Investigations of Oleic Acid Separation from Olive Oil and Olive Mill Wastewater: A comparative study

Mechanism of surface modification of jute fibers (JFs) by different... | Download Scientific Diagram

EP0294010A1 - Process and apparatus for the continuous production of transparent soap - Google Patents

Statement I: A triester of glycerol with stearic acid on boiling with `Aq`. `NaOH` gives solid cake - YouTube

Write a chemical equation for the reaction that occurs between stearic acid and triethanolamine under the conditions of the experiment. How does the product of this reaction promote the formation of the

Statement I: A triester of glycerol with stearic acid on boiling with `Aq`. `NaOH` gives solid cake - YouTube