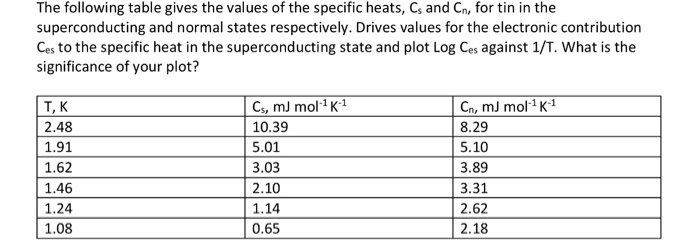
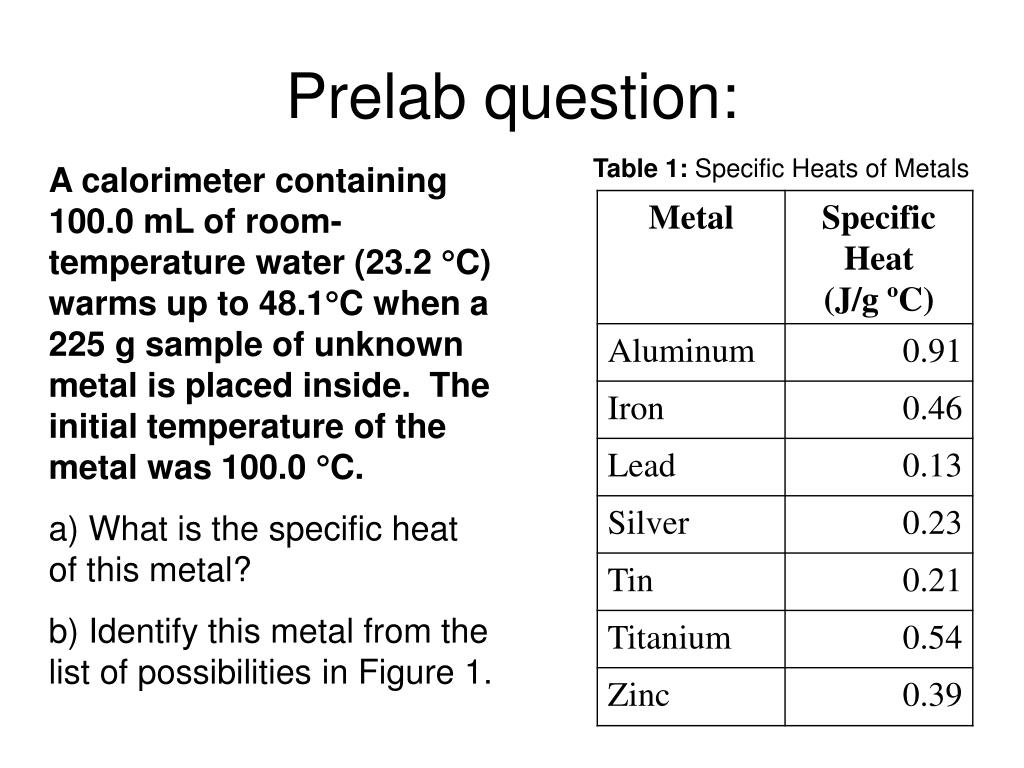
SOLVED: Table 1. Specific heat capacities and densities of common metals Metal Specific Heat Capacity 1.g deg Density (gicm ' ) Iron 0.45 7.87 Aluminum 0.91 2.70 Lead 0.13 11.36 Copper 8.96 Zinc 7.13 Tin 0.21 7.28 Gold 19.32
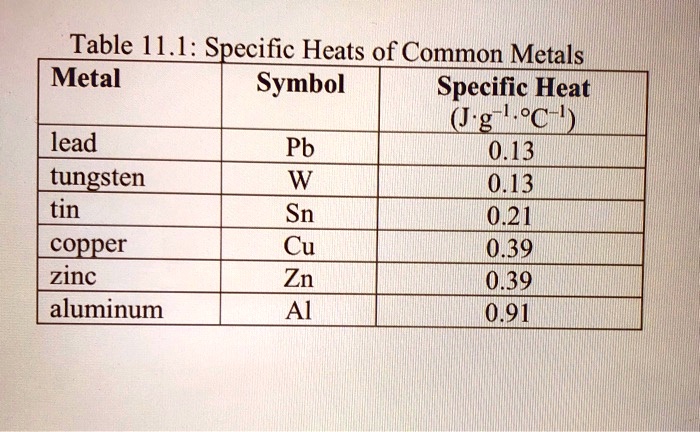
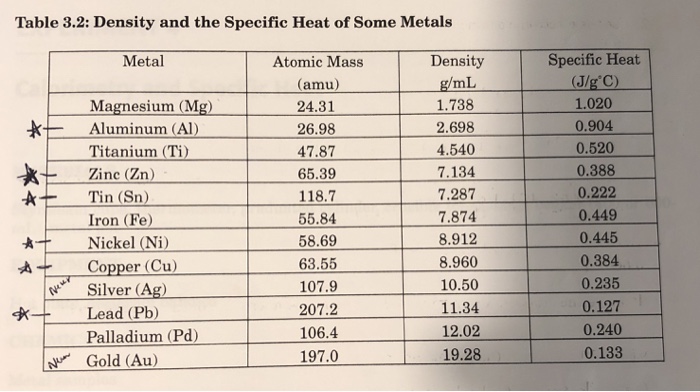
SOLVED: Table14.l: Specific Heats of Common Metals Metal Symbol Specific Heat (J.g 'C-I) lead 0.13 Pb tungsten 0.13 tin Sn 021 copper Cu 0.39 zinc 0.39 aluminum Al 0.91
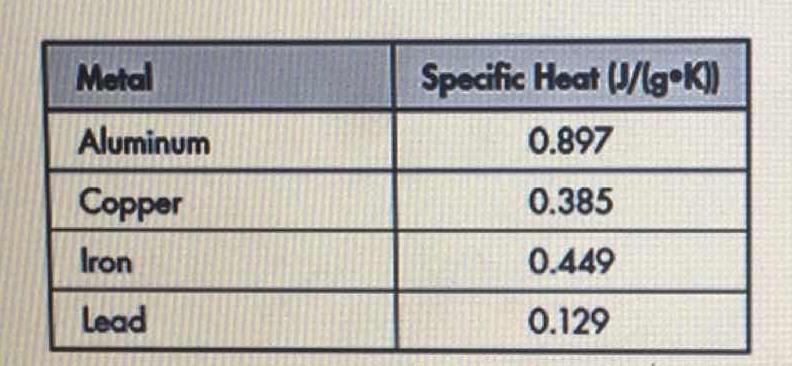
The table below shows the specific heats of several metals. The temperature of a 15-g sample of an unknown metal increases from 20.0 C to 30.0 C when it absorbs 67.5 J

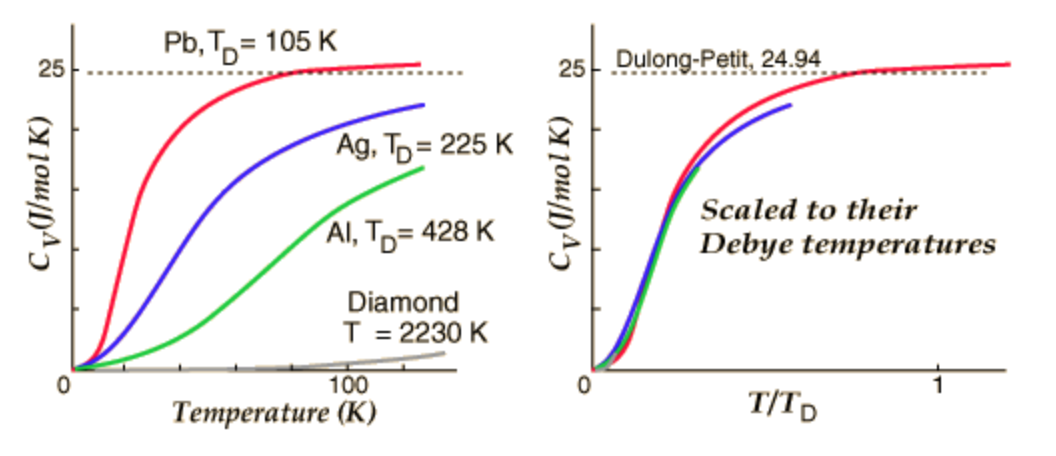
Compare the specific heats of the metals with their atomic weights (i.e., molar masses). Do you see any trends? Make a plot to show this relationship. What does the plot reveal?
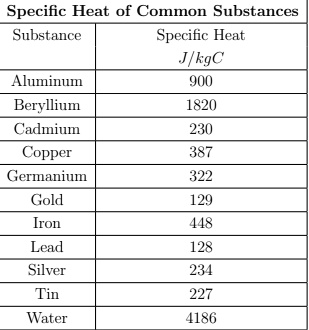
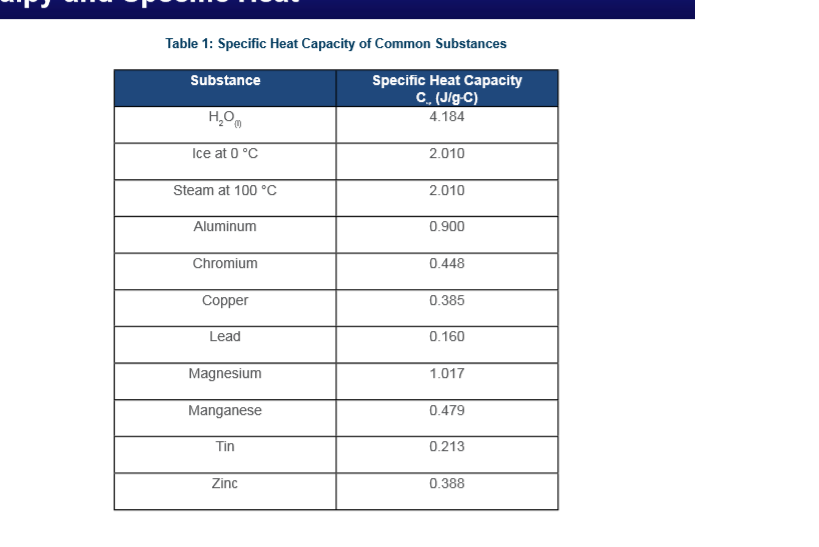
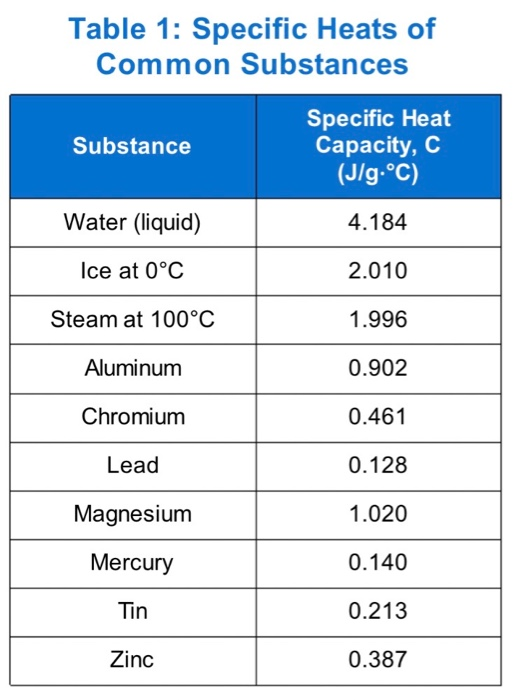
SOLVED: Specific Heat of Common Substances Substance Specific Heat T/kgC Aluminum Y(U Beryllium 1820 Cadmium 230 Copper 387 Germanium 322 Gold [ron 2 Lead Silver Tin 227 Water 4186