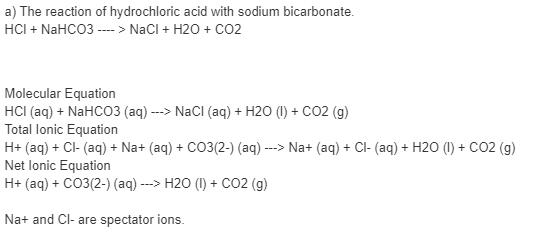
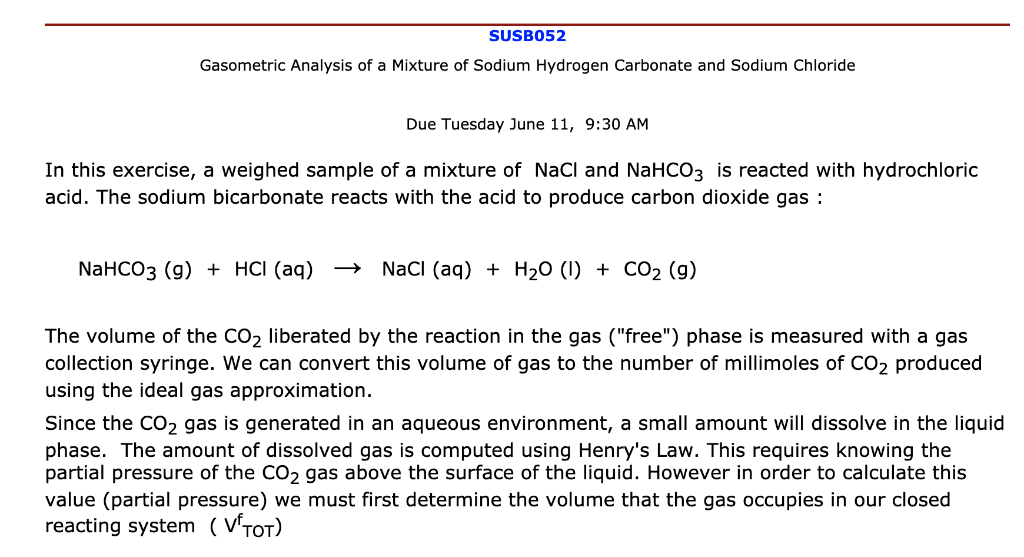
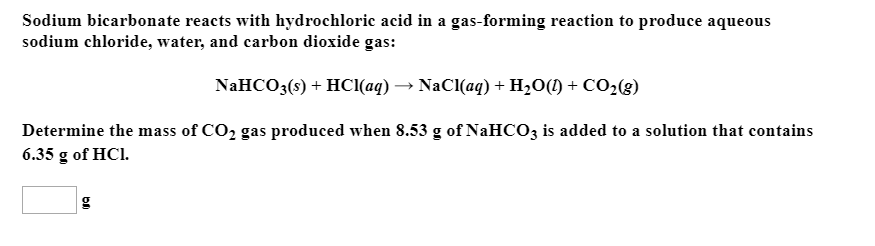
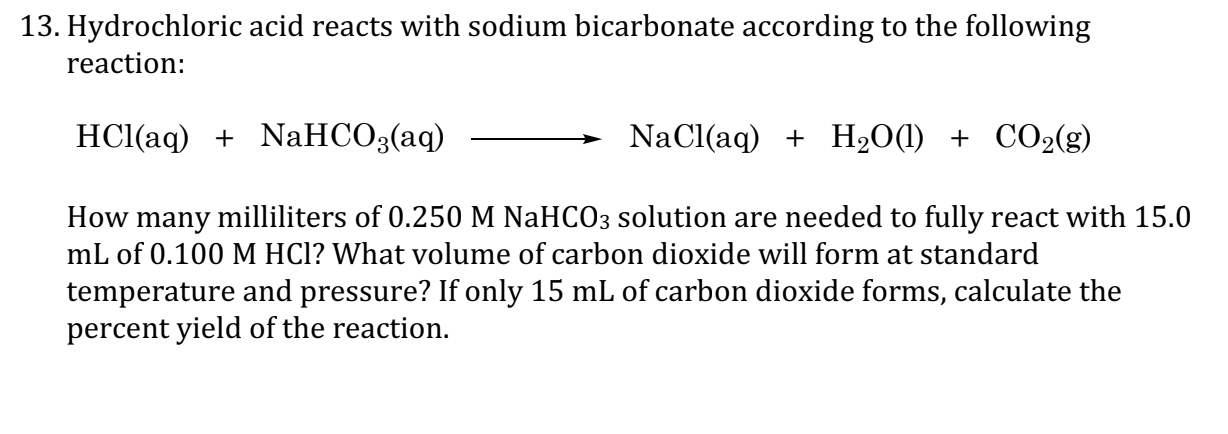Write an equation showing why the aspirin precipitates from when HCl is added to the saturated NaHCO3 solution. | Homework.Study.com

Sodium Bicarbonate and Hydrochloric Acid Stoichiometry Lab NaHCO 3 + HCl Core II Preview. - ppt download

SOLVED: Reaction of sodium bicarbonate with hydrochloric acid: 5 . NaHCO;() HClaq) NaCl(aq) + H,O() + COz(g) Formula: Ionic: Ner-lonic:

How to Balance NaHCO3 + HCl = NaCl + CO2 + H2O (sodium bicarbonate plus hydrochloric acid) - YouTube

Sodium Bicarbonate + Hydrochloric Acid - Balanced Molecular Equation, Complete and Net Ionic Equatio | Quizalize

How to Balance NaHCO3 + HCl = NaCl + CO2 + H2O (sodium bicarbonate plus hydrochloric acid) - YouTube

OneClass: Sodium bicarbonate is used to neutralize the remaining HCl at the end of the reaction. The ...

Two compounds X and Y, when dissolved in water, produced sodium bicarbonate. If hydrochloric acid is added to compound 'X', compound Y is liberated. Identify X and Y.
i) Sodium thiosulphate is reacted with dilute hydrochloric acid (ii) Calcium bicarbonate reacts with dilute hydrochloric acid - Sarthaks eConnect | Largest Online Education Community

Sodium bicarbonate on heating decomposes to form sodium carbonate, CO2 and water. If 0.2 moles of sodium bicarbonate is completely decomposed, how many moles of sodium carbonate is formed?



![MCQ] Sodium hydrogen carbonate when added to acetic acid evolves gas MCQ] Sodium hydrogen carbonate when added to acetic acid evolves gas](https://d1avenlh0i1xmr.cloudfront.net/24d7669e-1012-43e2-9ede-446e6d9b029d/reaction-of-sodium-hydrogen-carbonate-with-acetic-acid-01.jpg)