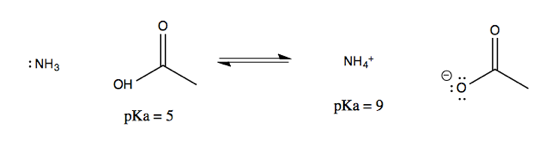
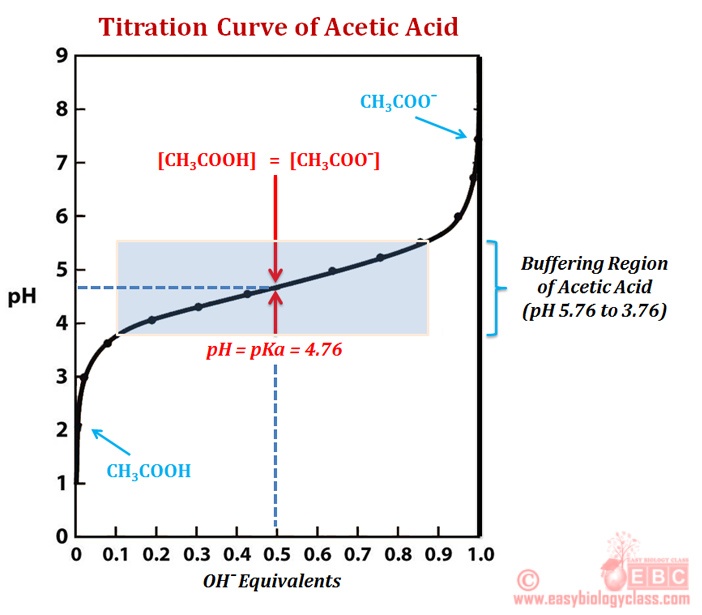
Match the List - I (solution of salts) with List - II (pH of the solution) and select the correct answer using the codes given below the lists:List - IList - IIA.

Experimental pKa values and structures of the conformers of acetic,... | Download Scientific Diagram

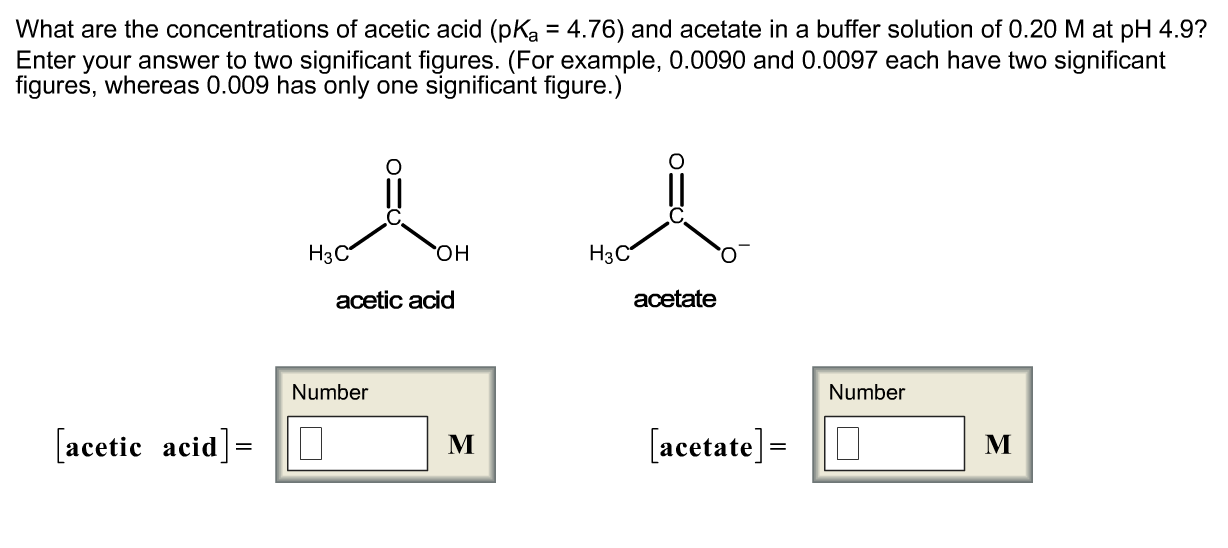
OneClass: What are the concentrations of acetic acid (pKa = 4.76) and acetate in a buffer solution of...

SOLVED: Calculate the ratio of acetic acid to acetate concentration needed to prepare a buffer with a pH of 5.80. Acetic acid pka = 4.70.
The pKa value of acetic acid is 4.7447 at 25°C. How would you obtain a buffer of acetic acid and sodium acetate with pH = 4? - Sarthaks eConnect | Largest Online Education Community

Acid Dissociation Constant (pKa) of Common Monoethylene Glycol (MEG) Regeneration Organic Acids and Methyldiethanolamine at Varying MEG Concentration, Temperature, and Ionic Strength | Journal of Chemical & Engineering Data

Chemistry :-The pkb for dimethylamine and pka for acetic acid are 3.27 and 4.77. find pH #neet2021 - YouTube

The dependency of the pKa of acetic acid on the ionic strength, at 18... | Download Scientific Diagram

Experimentation with different thermodynamic cycles used for pKa calculations on carboxylic acids using complete basis set and Gaussian‐n models combined with CPCM continuum solvation methods - Liptak - 2001 - International Journal

OneClass: What concentrations of acetic acid (pKa = 4.76) and acetate would be required to prepare a ...