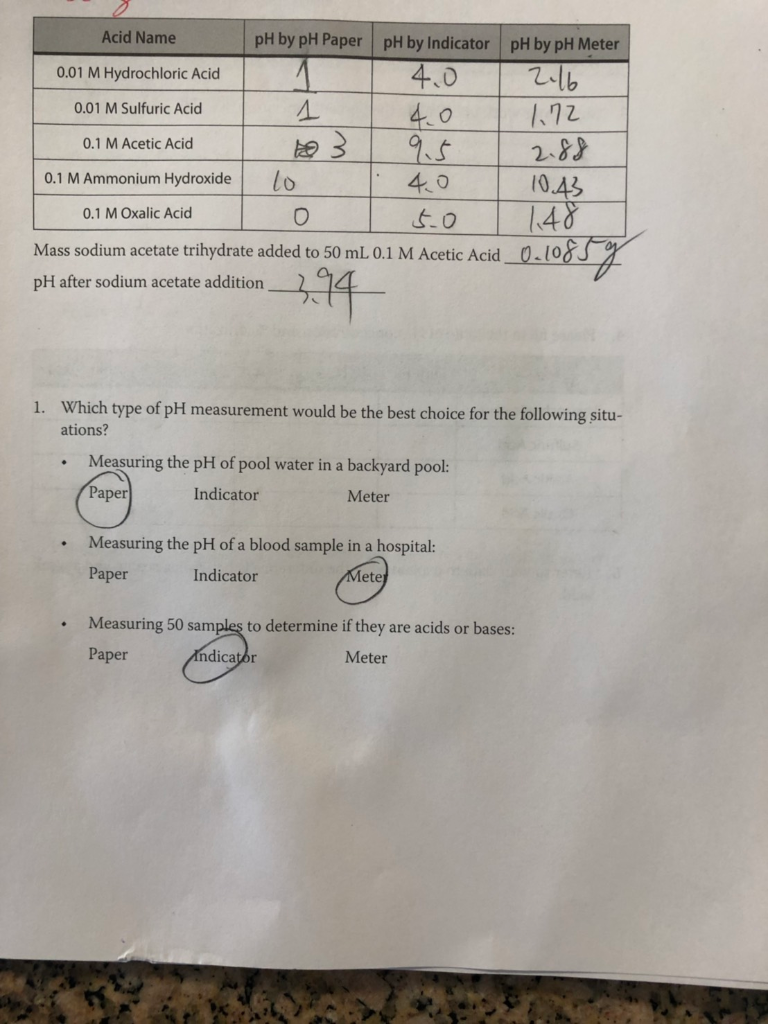
Calculate ph of 0.1m solution of acetic acid if degree of dossocuation of the acid us 0.0132 - Brainly.in

What is the tenths digit in the decimal representation of a certain number?(1) The number is less than 1/3 .(2) The number is greater than 1/4
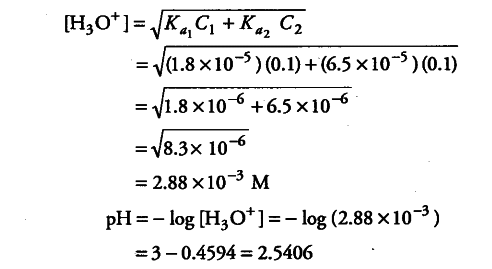
Calculate the pH of a solution containing 0.1 M acetic acid and 0.1 M benzoic acid - CBSE Class 11 Biology - Learn CBSE Forum

01M acetic acid solution is titrated against 0.1M NaOH solution. What would be the difference in pH between 1/4 and 3/4 stages of neutralisation of acid ?

when 10 ml of 0 1 M acetic acid ( pka = 50) titrated against 10 ml of 01 - Chemistry - Equilibrium - 10271133 | Meritnation.com

SOLVED: Suppose you made buffer solution that 0.050 M both HC2H302 and NaC2H30z Would the pH changes resulting from the addition of O,! M HCI solution to this buffer solution be the

50 mL of 0.1 M solution of sodium acetate and 50 mL of 0.01 M acetic acid are mixed. The pKa of acetic acid is 4.76. The pH of the buffer solution is:

Calculate the pH of 0.1 M acetic acid solution if its dissociation constant is 1.8 × 10^-5 . If 1 litre of this solution is mixed with 0.05 mole of HCl , what will be pH of the mixture?
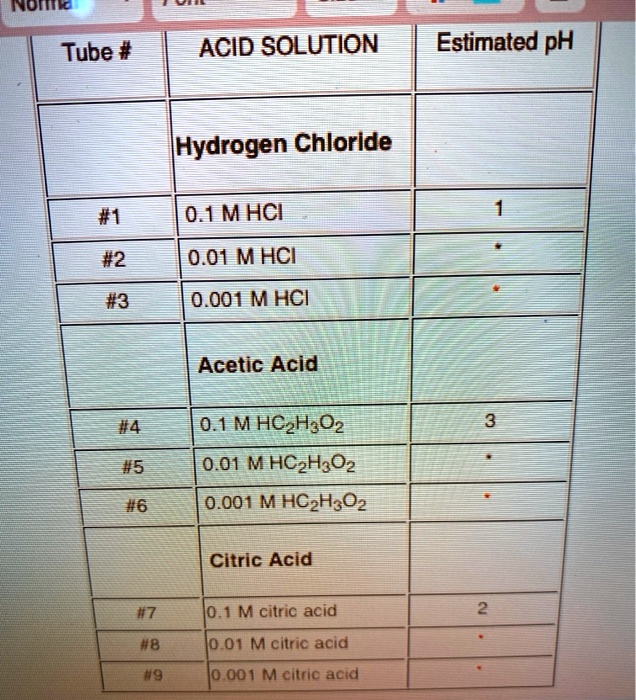
SOLVED: Inomo Tube # ACID SOLUTION Estimated pH [Hydrogen Chloride #1 0.1MHCI #2 #3 0.01 MHCI 0.001 MHCI Acetic Acid #4 0.1MHCzH;Oz 0.01 M HCzH3Oz 0.001 M HCzH3Oz #5 #6 Citric Acid