Microscale pH variations during drying of soils and desert biocrusts affect HONO and NH3 emissions | Nature Communications
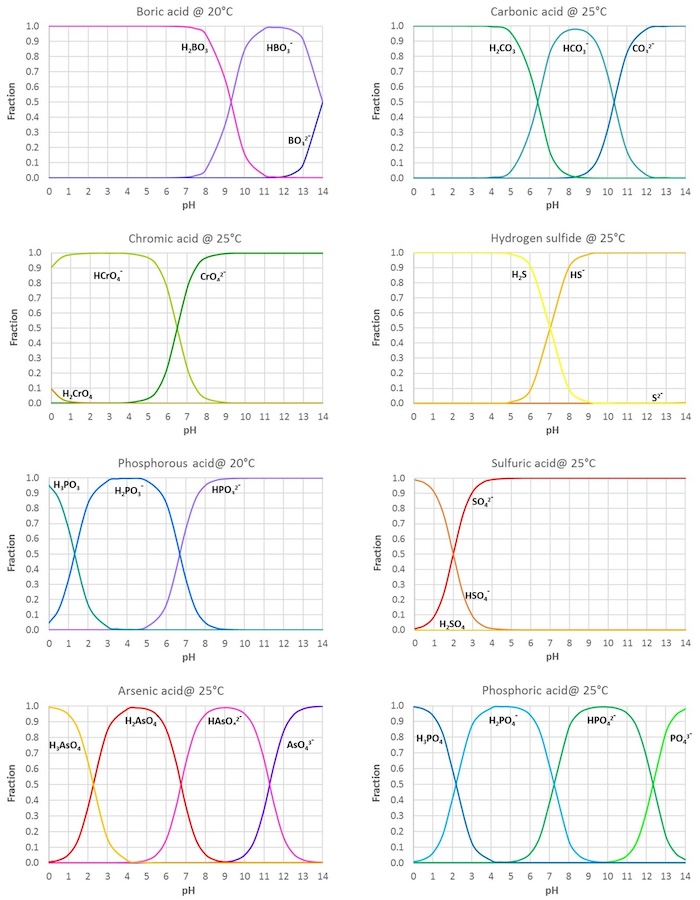
SOLVED: Which acid is the best choice to create a buffer with pH == 2.15? chlorous acid (HC1O2), pKa = 1.95 nitrous acid (HNO2), pKa = 3.34 acetic acid (HCHsCO2) , pKa =

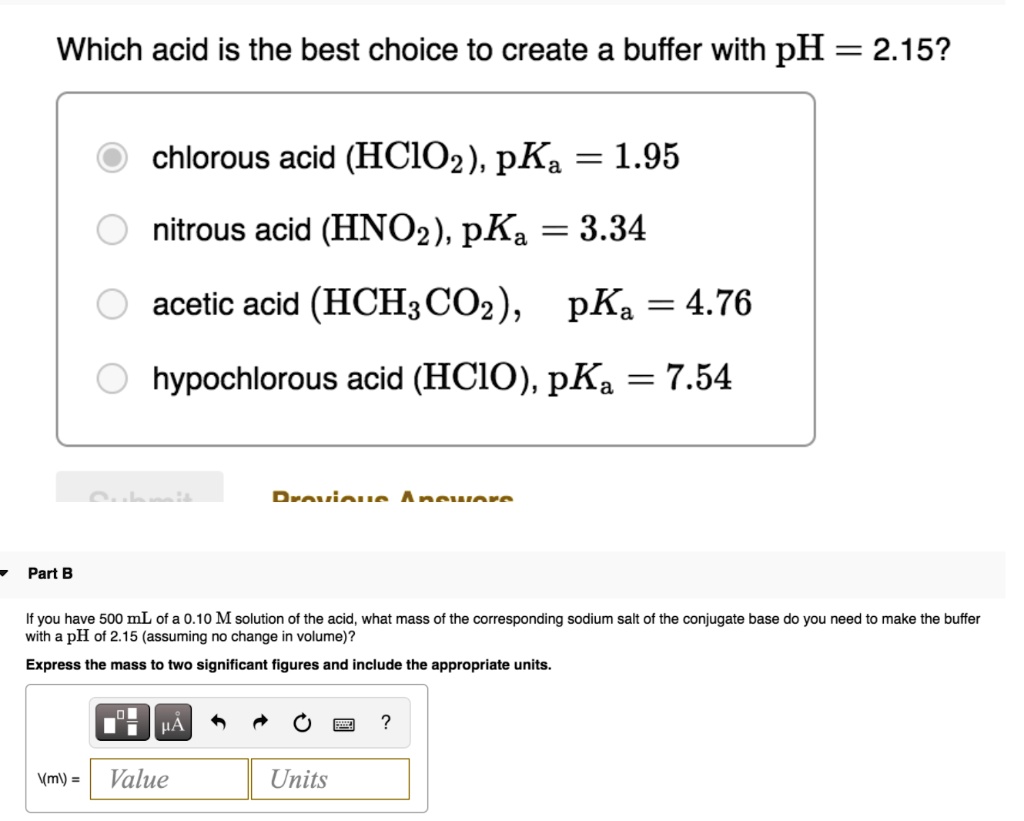
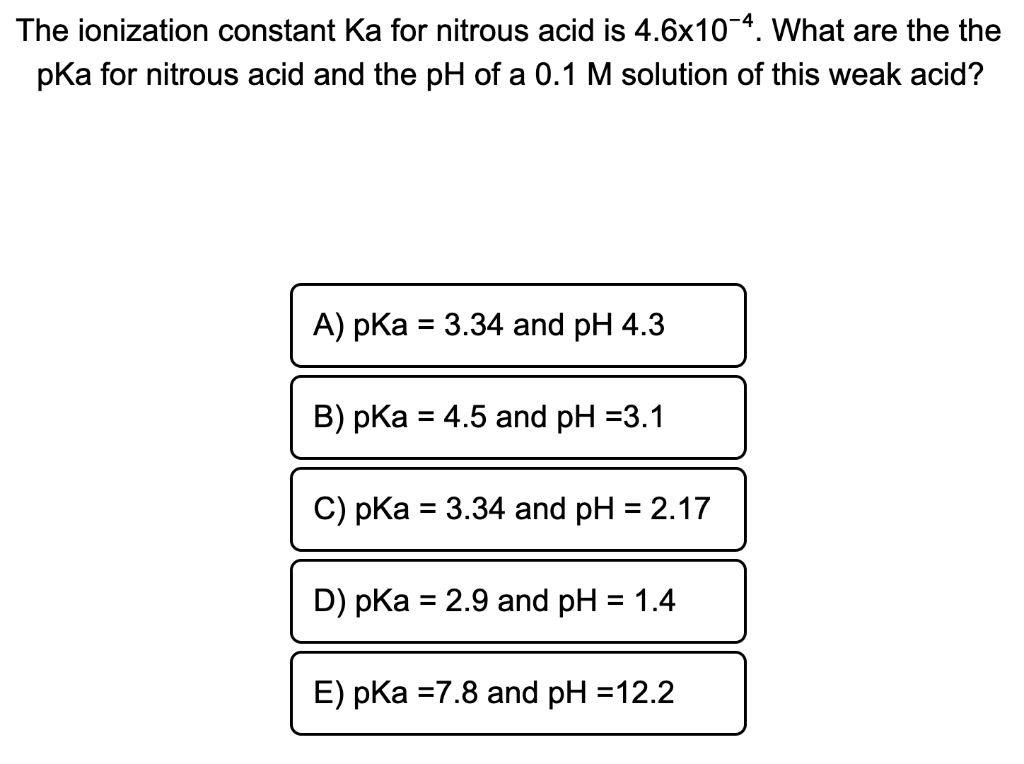
The ionization constant of nitrous acid is 4.5 × 10^-4 . Calculate the pH of 0.04 M sodium nitrite solution and also its degree of hydrolysis.
![PDF] UV-visible spectrum of nitrous acid in solution: pKa determination and analytical applications | Semantic Scholar PDF] UV-visible spectrum of nitrous acid in solution: pKa determination and analytical applications | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/7d7622ce83d25c305576ab00e3af3523a05148a3/2-Table1-1.png)
PDF] UV-visible spectrum of nitrous acid in solution: pKa determination and analytical applications | Semantic Scholar
![PDF] UV-visible spectrum of nitrous acid in solution: pKa determination and analytical applications | Semantic Scholar PDF] UV-visible spectrum of nitrous acid in solution: pKa determination and analytical applications | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/7d7622ce83d25c305576ab00e3af3523a05148a3/3-Figure1-1.png)
PDF] UV-visible spectrum of nitrous acid in solution: pKa determination and analytical applications | Semantic Scholar

SOLVED: 4J-1 113 The pH value ofa mixture Buffer of nitrous acid (HNOZ) Pka = 3.35 At a concentration of 0.12 M and sodium nitrite NaNO2 at a concentration of 0.15M Equal

Superoxide and Nitrous Acid Production from Nitrate Photolysis Is Enhanced by Dissolved Aliphatic Organic Matter | Environmental Science & Technology Letters


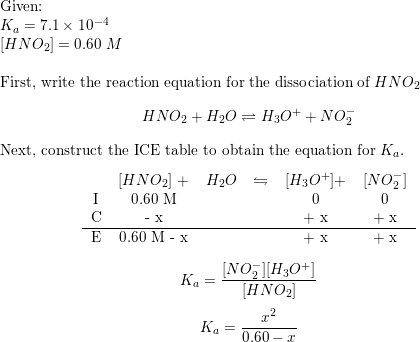
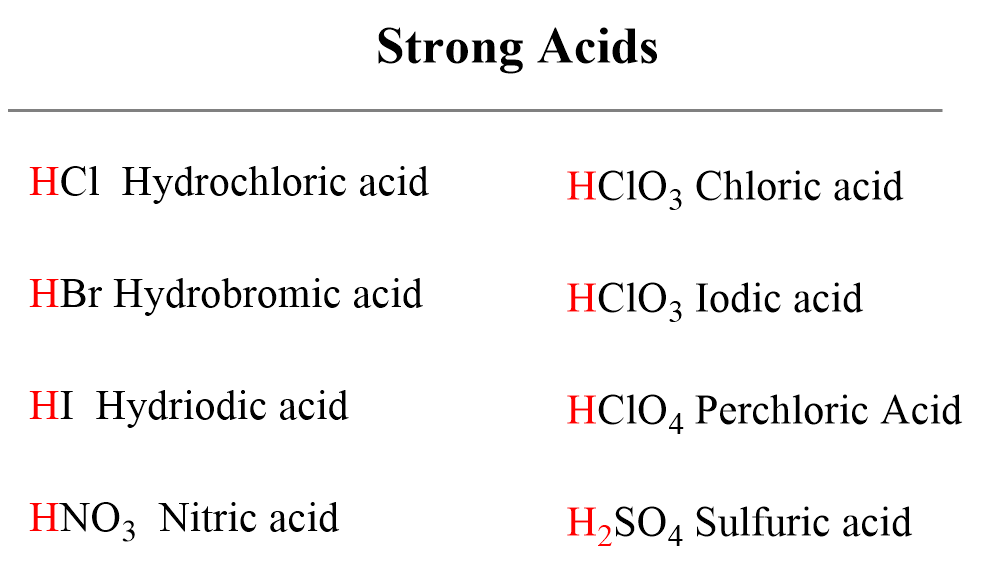

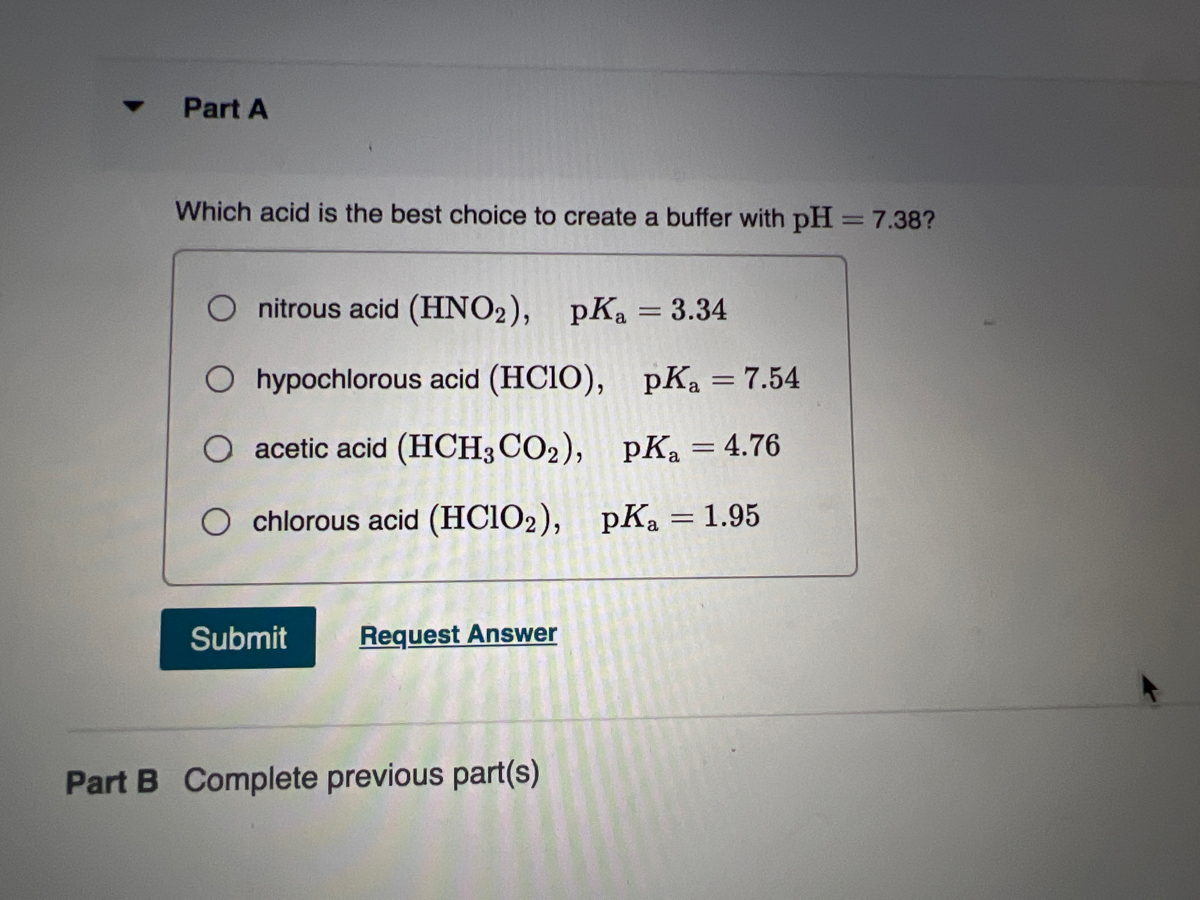

![ANSWERED] Use the pka table to select a base to depr... - Organic Chemistry ANSWERED] Use the pka table to select a base to depr... - Organic Chemistry](https://media.kunduz.com/media/sug-question/raw/80475089-1659894957.2799208.jpeg)