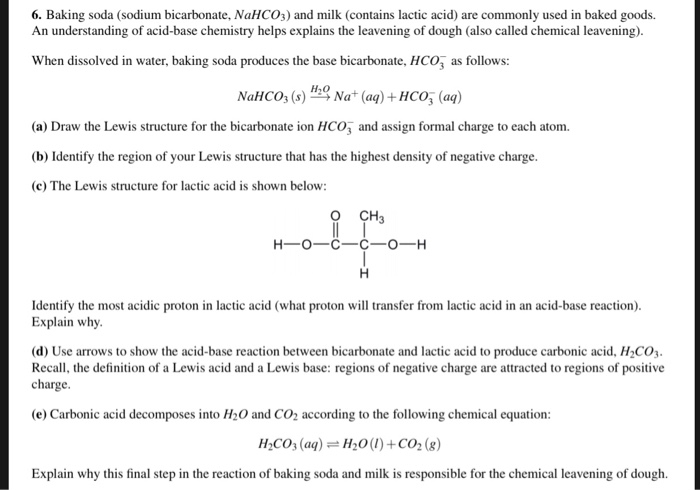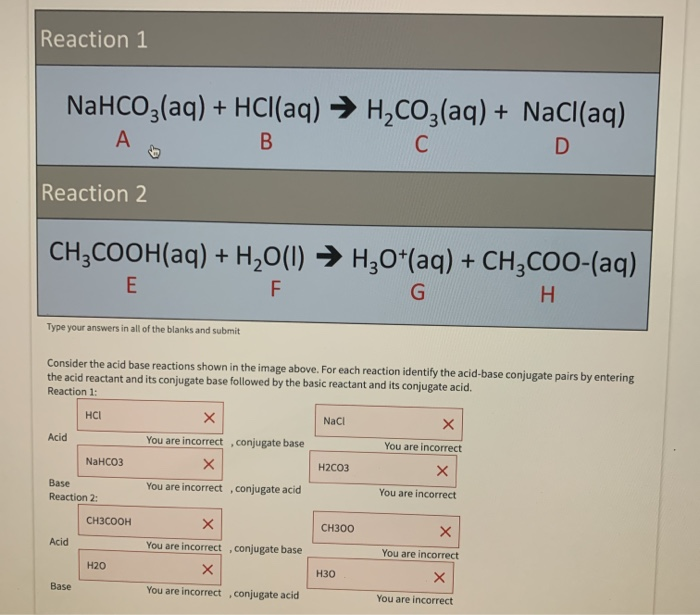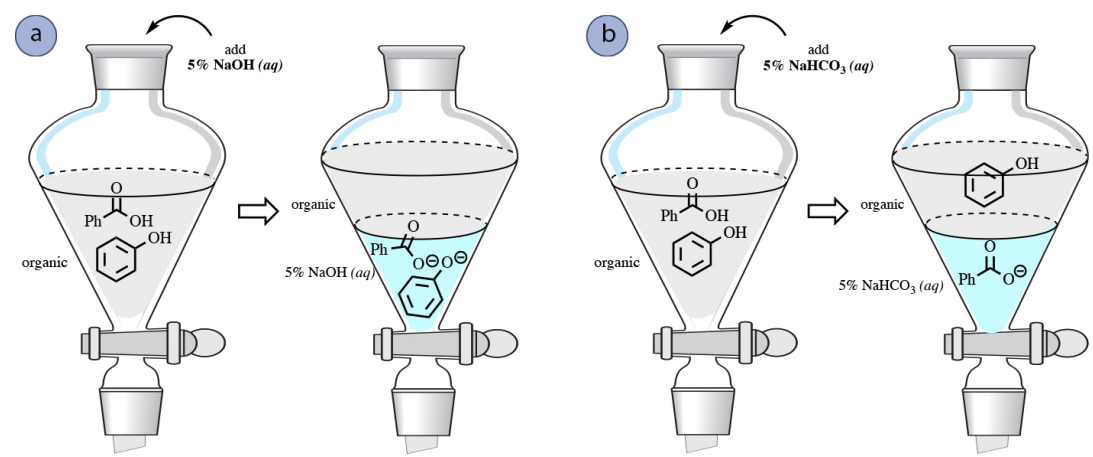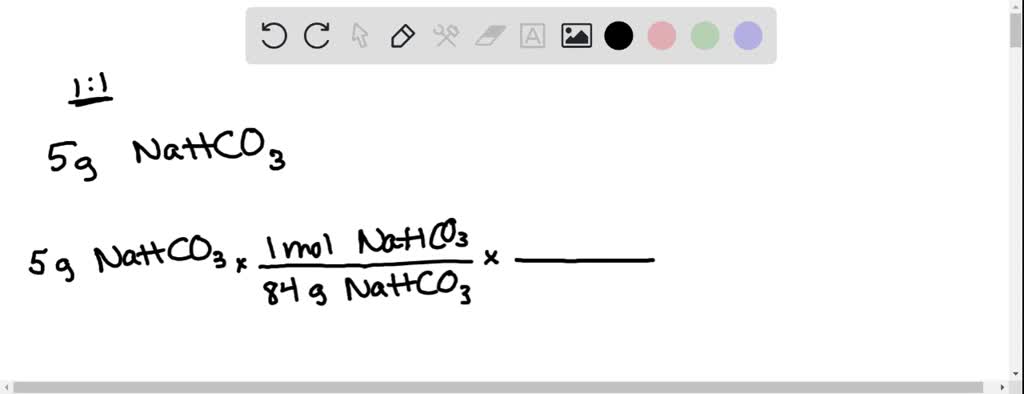
SOLVED: How many moles and grams of acetic acid would be required if 5 grams of sodium bicarbonate reacted completely with no unreacted acid or base (i.e. with an exact stoichiometric ratio?

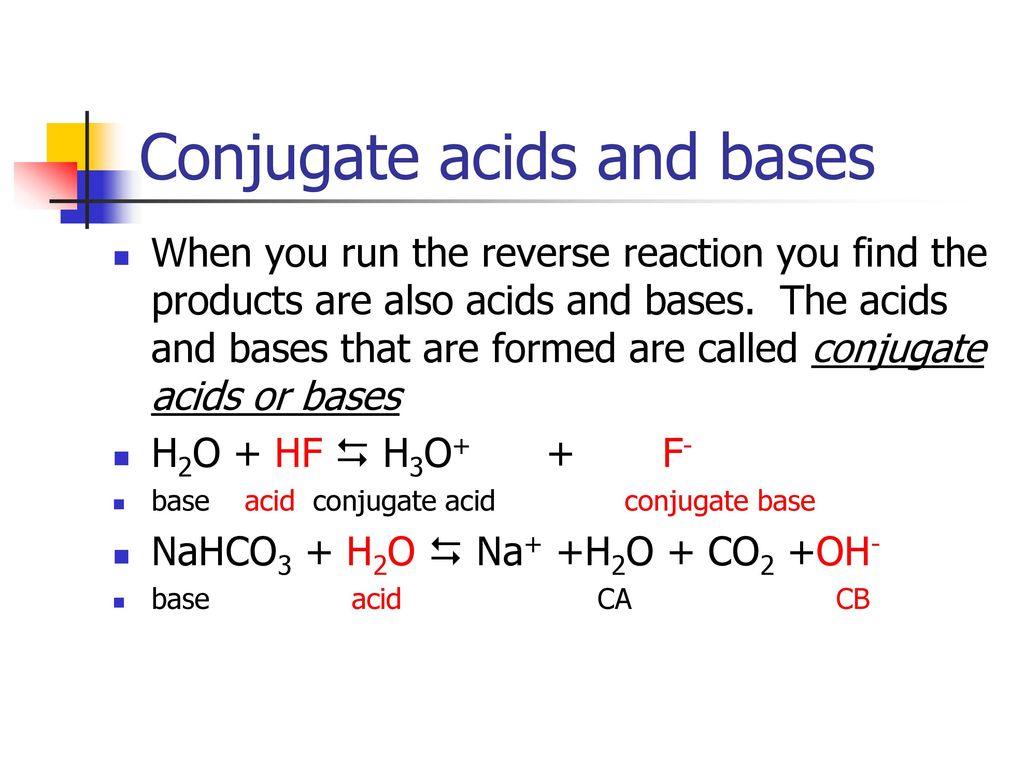
Question Video: Identifying the Salt Product of the Reaction between Ethanoic Acid and Sodium Bicarbonate | Nagwa

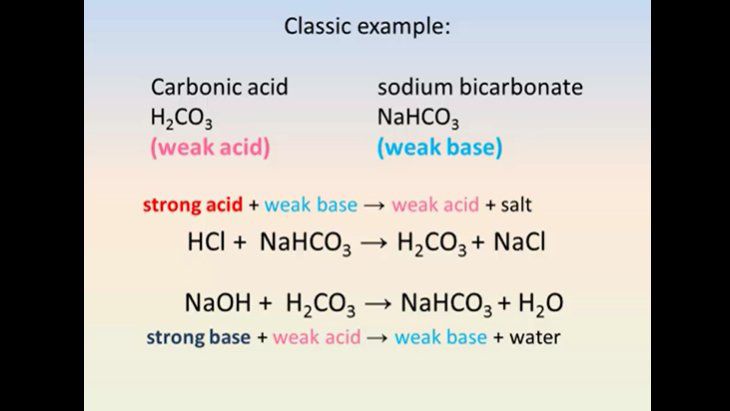
How to Balance NaHCO3 + HCl = NaCl + CO2 + H2O (sodium bicarbonate plus hydrochloric acid) - YouTube
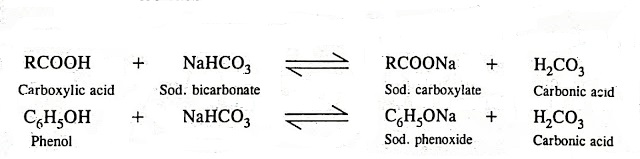
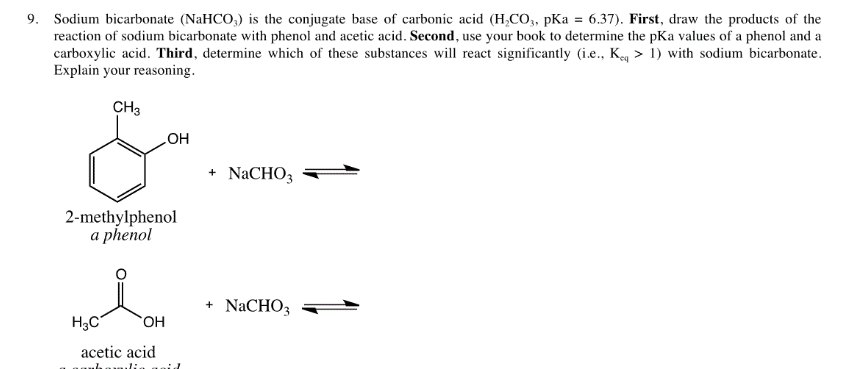
Welcome to Chem Zipper.com......: Why does aqueous sodium bicarbonate solution dissolve carboxylic acids but not phenol though they are also acidic ?