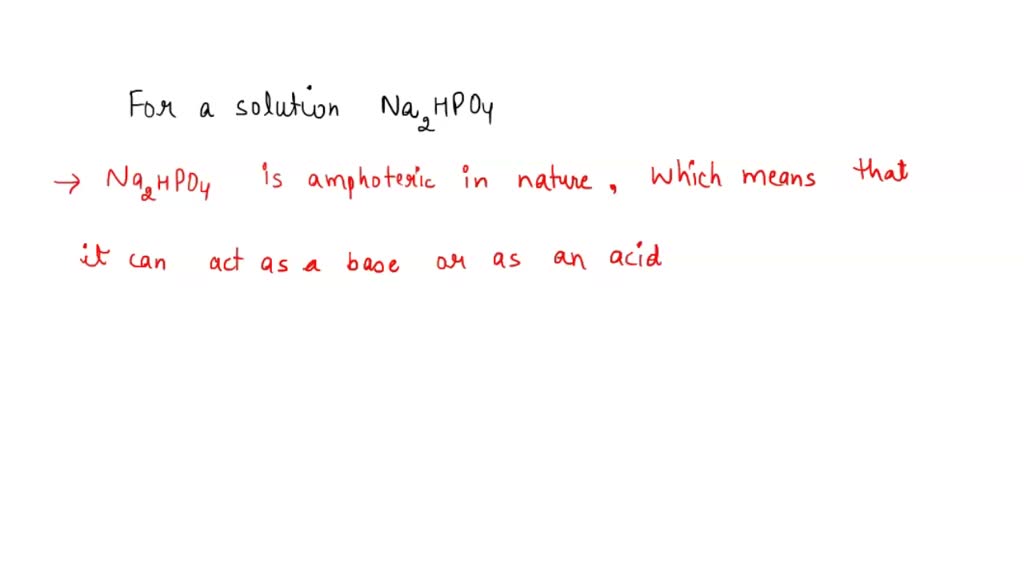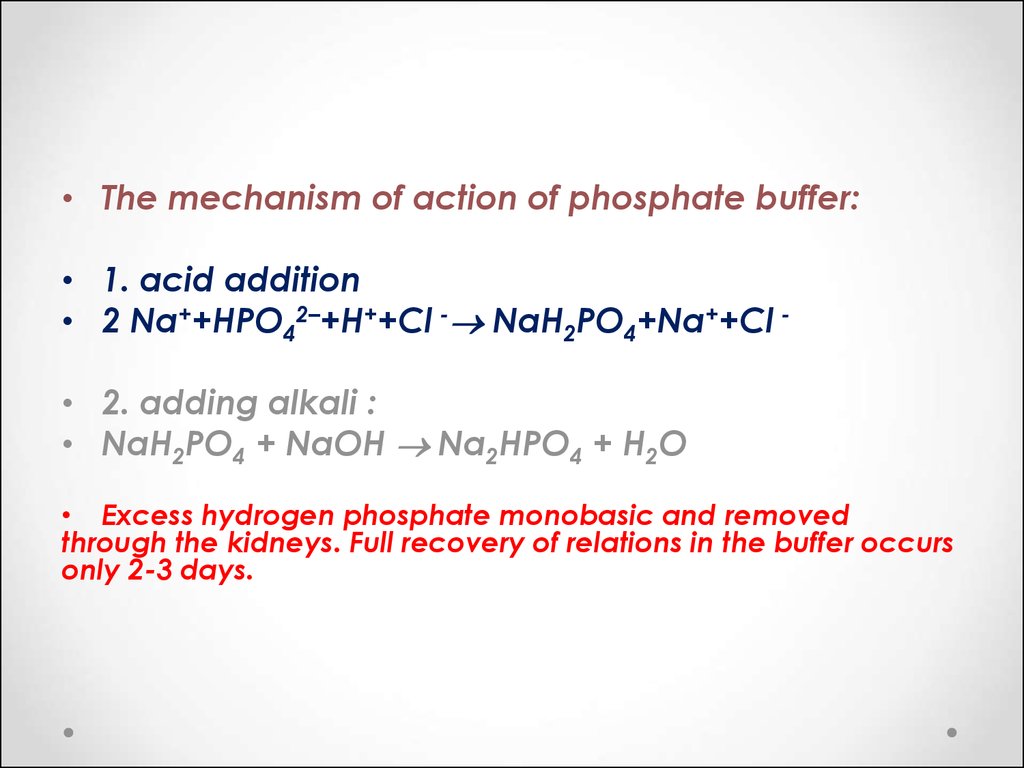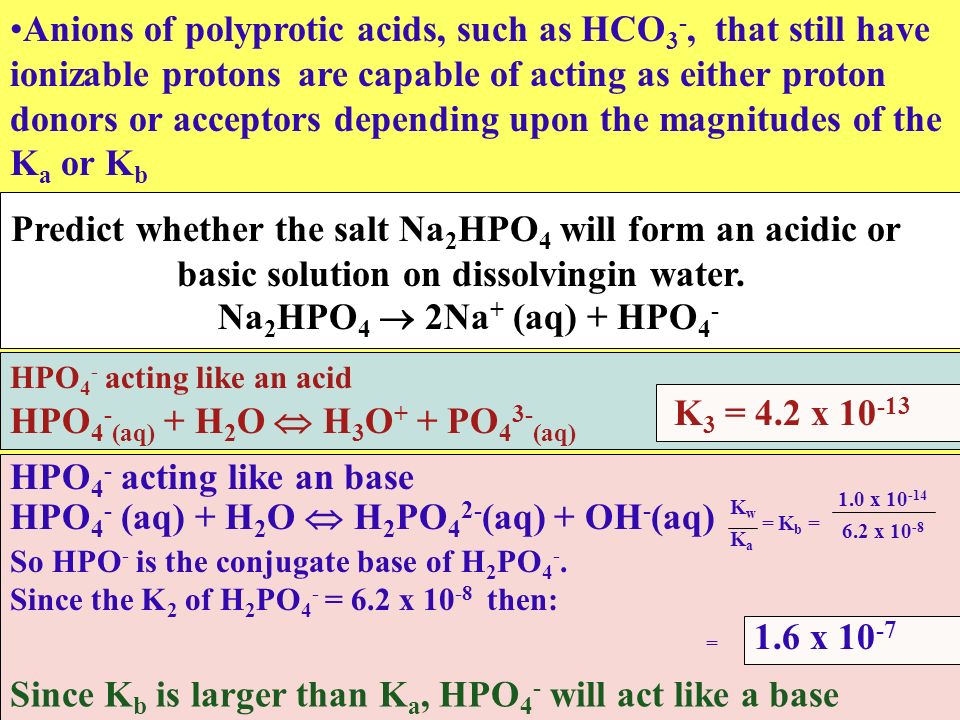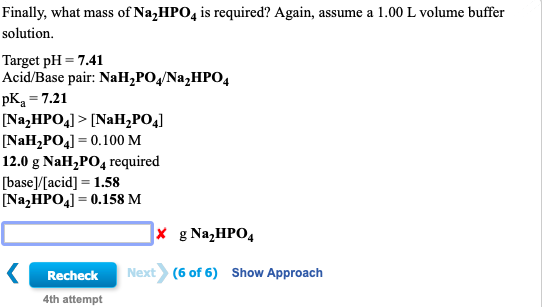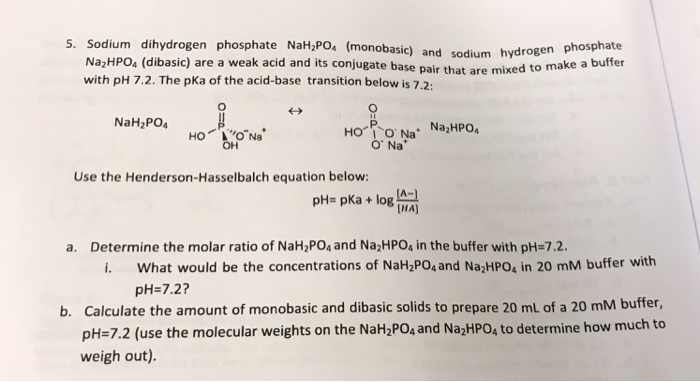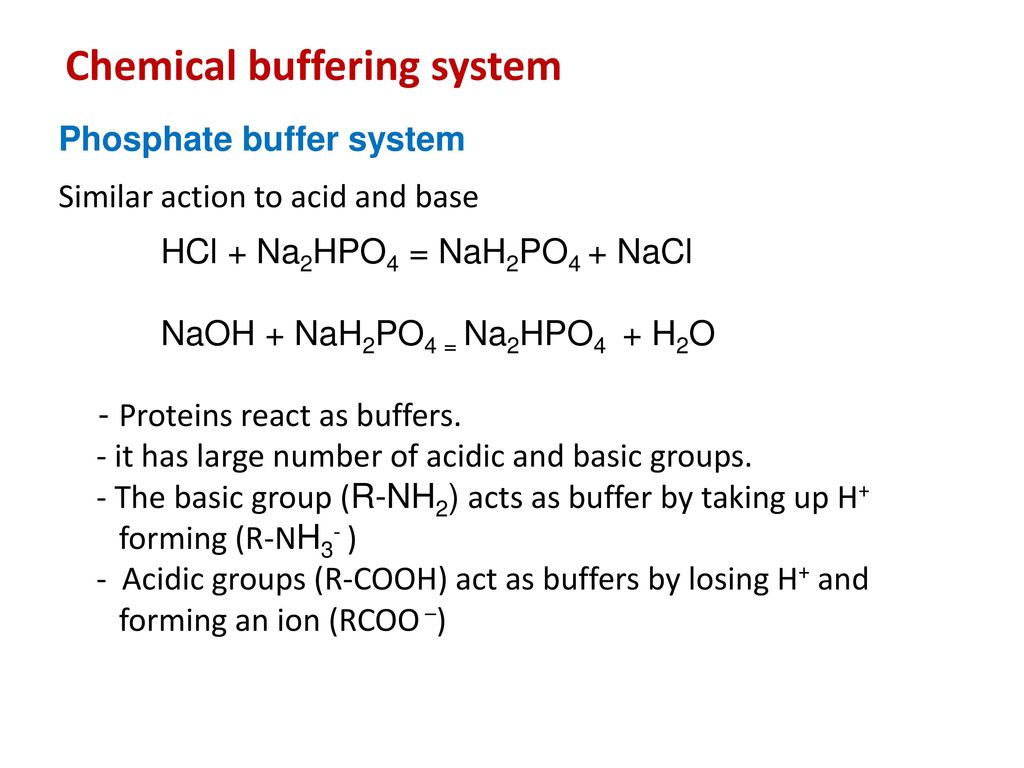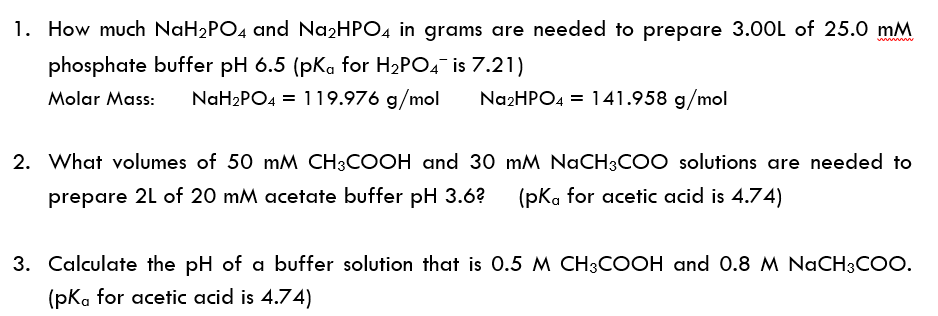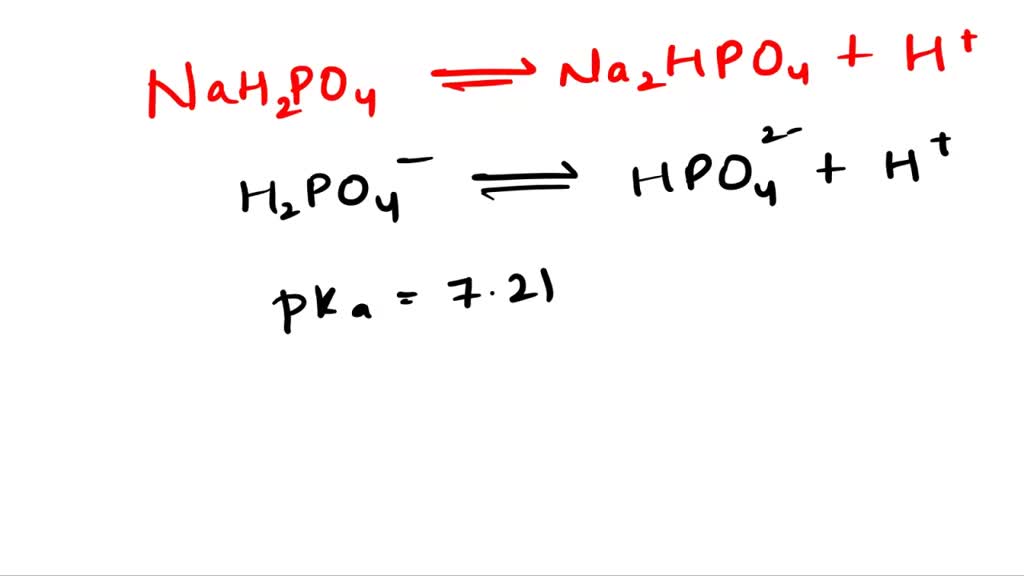
SOLVED: 1. Why is the equilibrium between the acid NaH2PO4, and its conjugate base Na2HPO4, a suitable buffer for maintaining intracellular pH (pH 6.9-7.3)?

A buffer solution 0.04 M in Na2HPO4 and 0.02 M in Na3PO4 is prepared. The electrolytic oxidation of 1.0 milli - mole of the organic compound RNHOH is carried out in 100
Q. The equivalent mass of H3PO4 (Molecular weight = 98 g/mol) and Na2HPO4 (Molecular weight = 142 g/mol) in the reaction are respectively : H3PO4 + 2NaOH → Na2HPO4 + 2H2O (1) 49, 142 (2) 49, 71 (3) 98, 71 (4) 98, 142
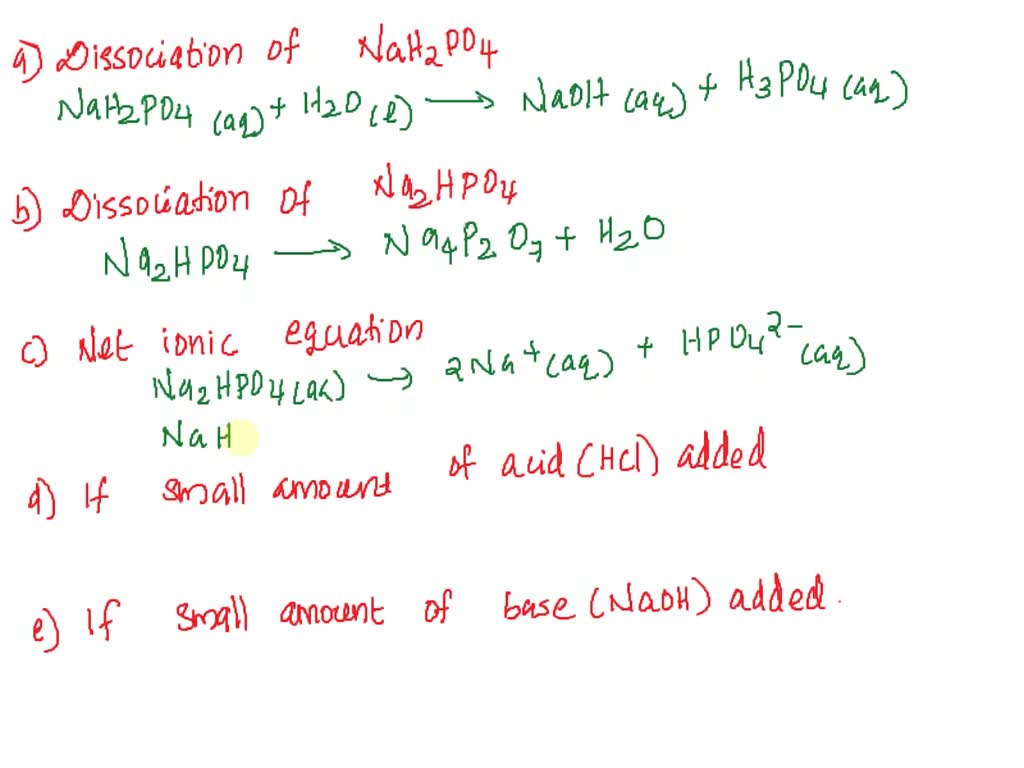
SOLVED: A buffer was made by mixing aqueous solutions of NaH2PO4 and Na2HPO4 together. This buffer is made by mixing two salts together. a. Write the balanced dissociation reaction for solid NaH2PO4
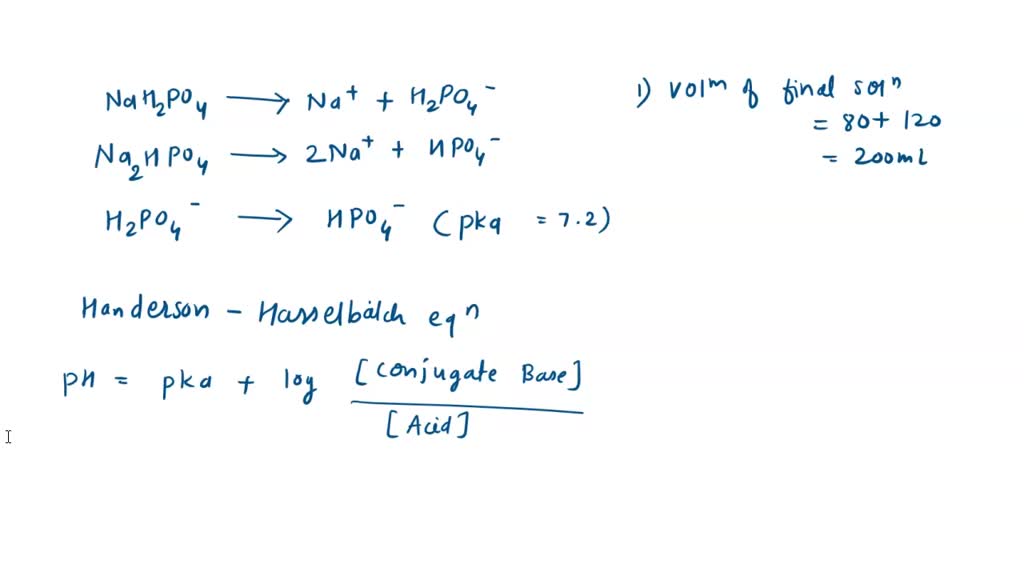
SOLVED: Calculate the pH of a buffer solution that is made by mixing 80 mL of 0.6 M NaH2PO4 with 120 mL of 0.8 M of Na2HPO4. Calculate the pH when 10


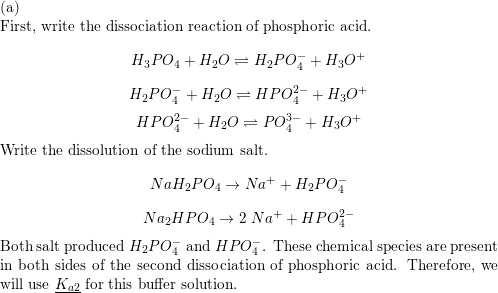

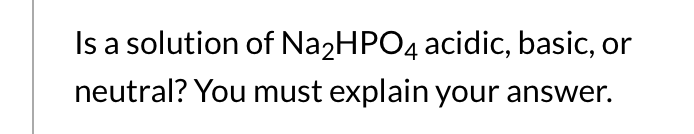



![An example of an acid salt is [CH(3)COONa//NaNO(3)//Na(2)HPO(4)//NaKCO(3)] An example of an acid salt is [CH(3)COONa//NaNO(3)//Na(2)HPO(4)//NaKCO(3)]](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/643651395_web.png)