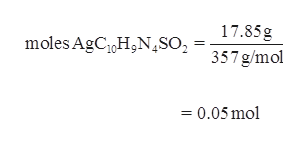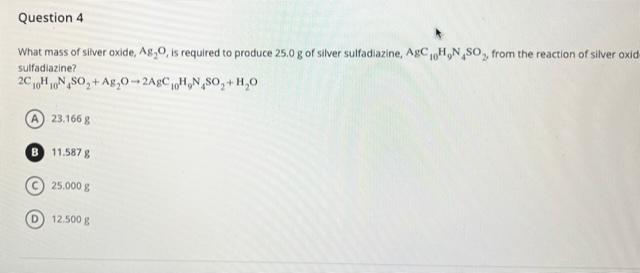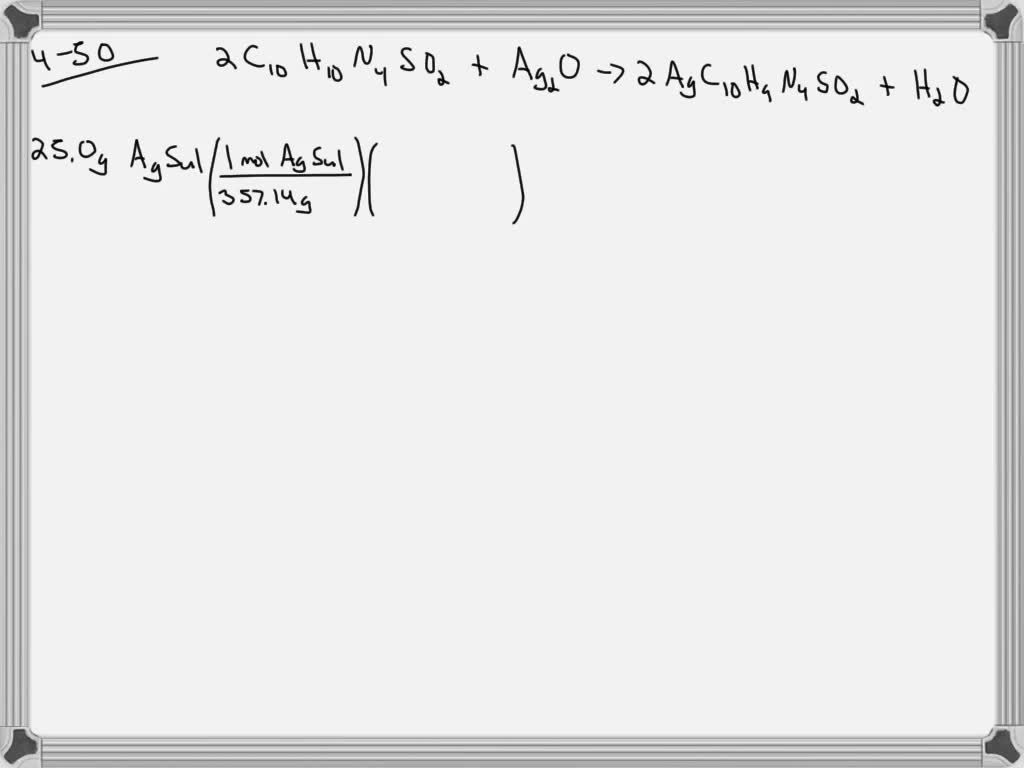
SOLVED:What mass of silver oxide, Ag2 O, is required to produce 25.0 g of silver sulfadiazine, AgC10 H9 N4 SO2, from the reaction of silver oxide and sulfadiazine? 2 C10 H10 N4
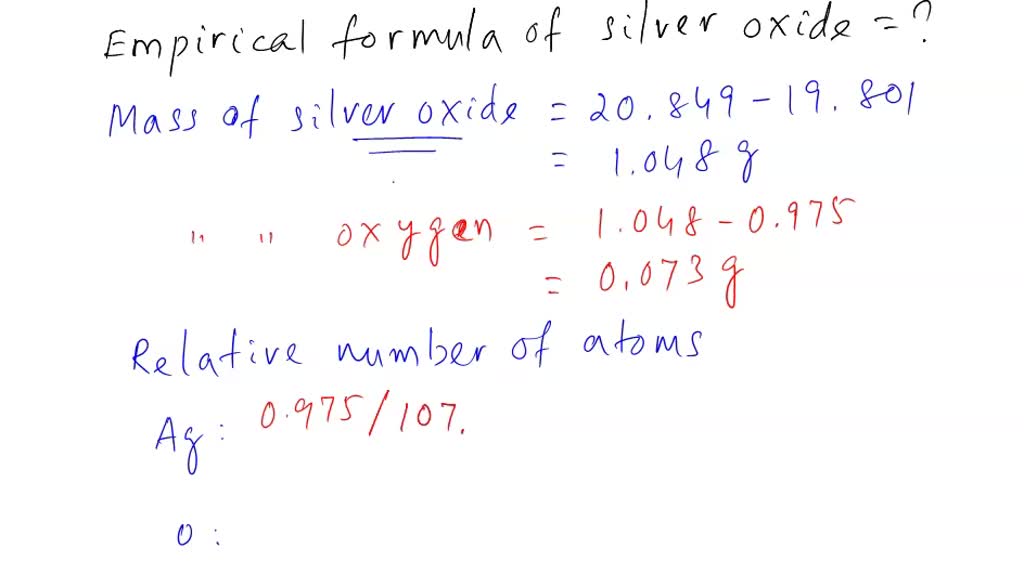
SOLVED: Challenge yowrself mnetal, forms an oxide with the formula M,Os This oxide contains 65.2 % of the metal by mass What'$ the Jdentity of the metal? Hint: To figure out the

SOLVED:What mass of silver oxide, Ag2 O, is required to produce 25.0 g of silver sulfadiazine, AgC10 H9 N4 SO2, from the reaction of silver oxide and sulfadiazine? 2 C10 H10 N4

Forum 5.docx - Chapter 7 50. What mass of silver oxide, Ag2O, is required to produce 25.0 g of silver sulfadiazine, AgC10H9N4SO2, from the reaction of | Course Hero
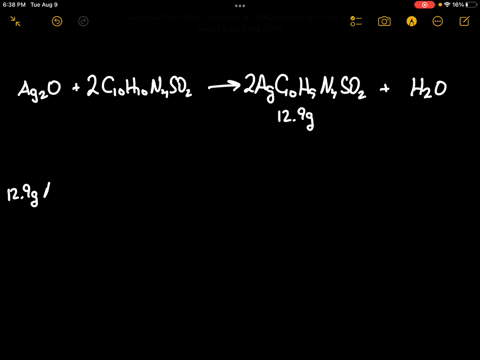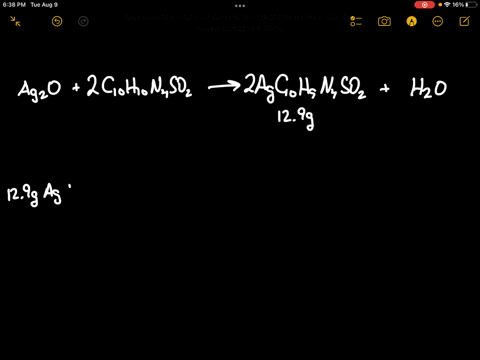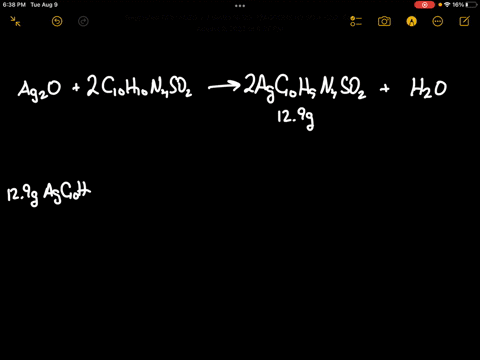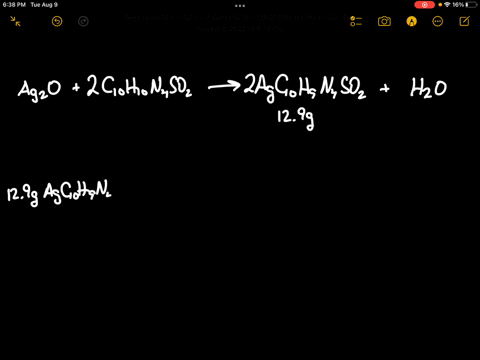
SOLVED: Silver oxide (Ag2O) reacts with sulfadiazine (C10H10N4SO2) to form silver sulfadiazine (AgC10H9N4SO2) and water. What mass of silver oxide is required to produce 12.9 g of silver sulfadiazine? g Ag2O
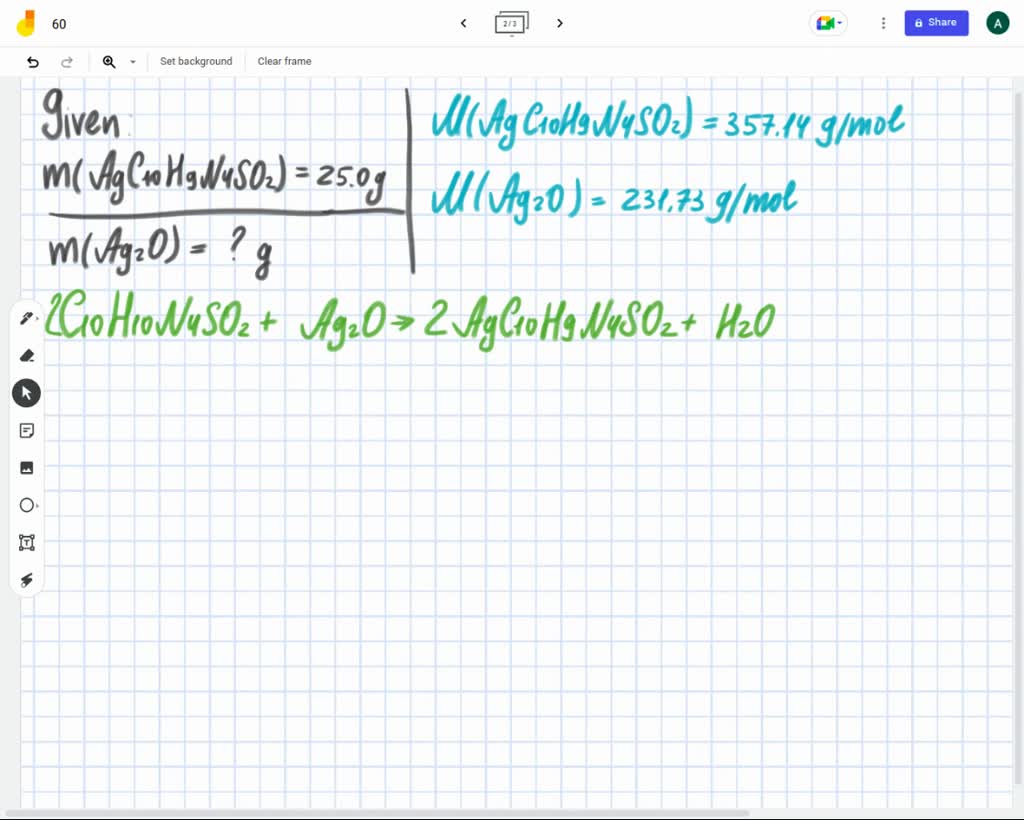
SOLVED: What mass of silver oxide, Ag2O, is required to produce 25.0 g of silver sulfadiazine, AgC10H9N4SO2, from the reaction of silver oxide and sulfadiazine?2C10 H10 N4 SO2 + Ag2 O ⟶

Forum 5.docx - Chapter 7 50. What mass of silver oxide, Ag2O, is required to produce 25.0 g of silver sulfadiazine, AgC10H9N4SO2, from the reaction of | Course Hero