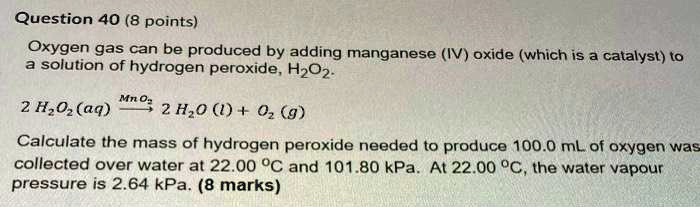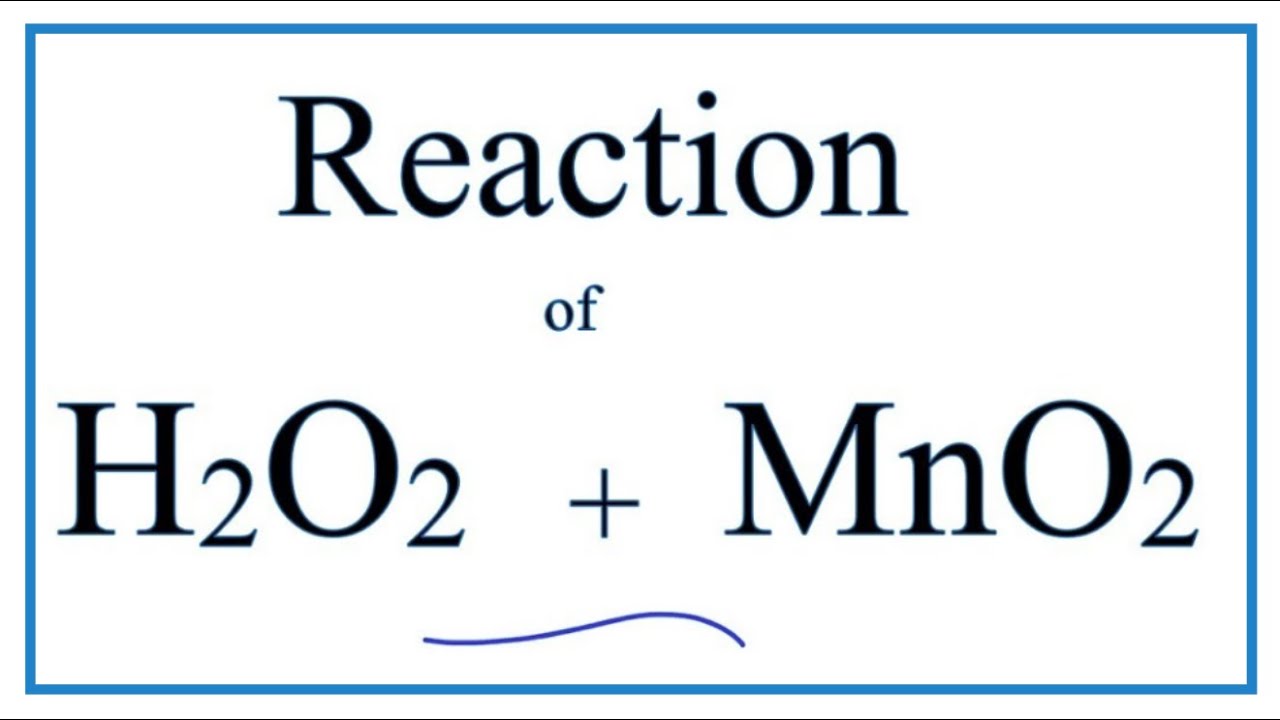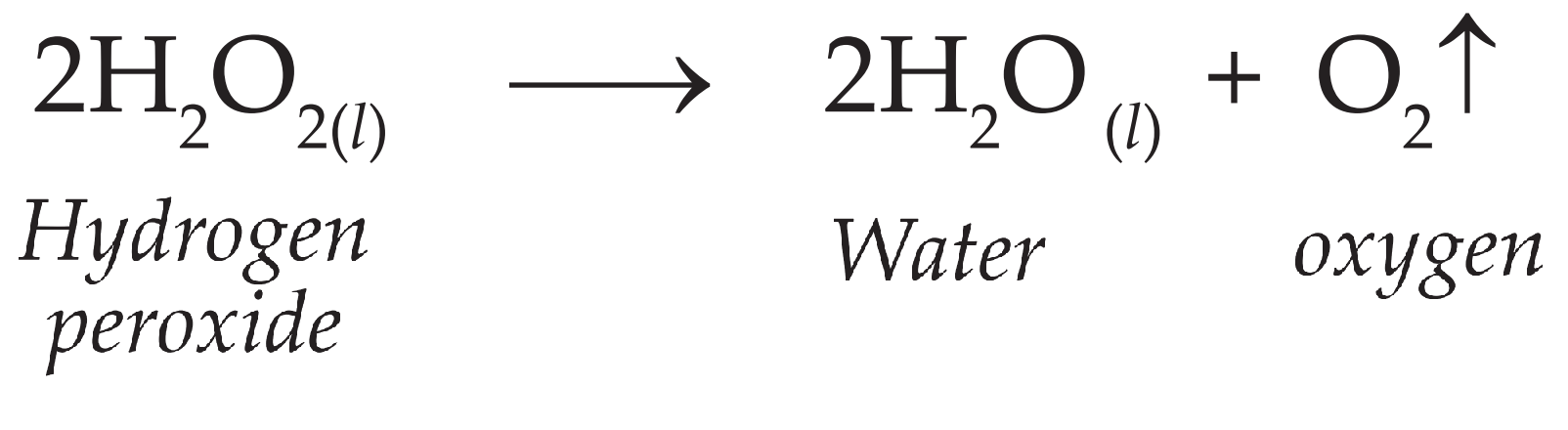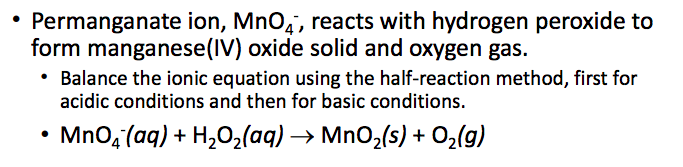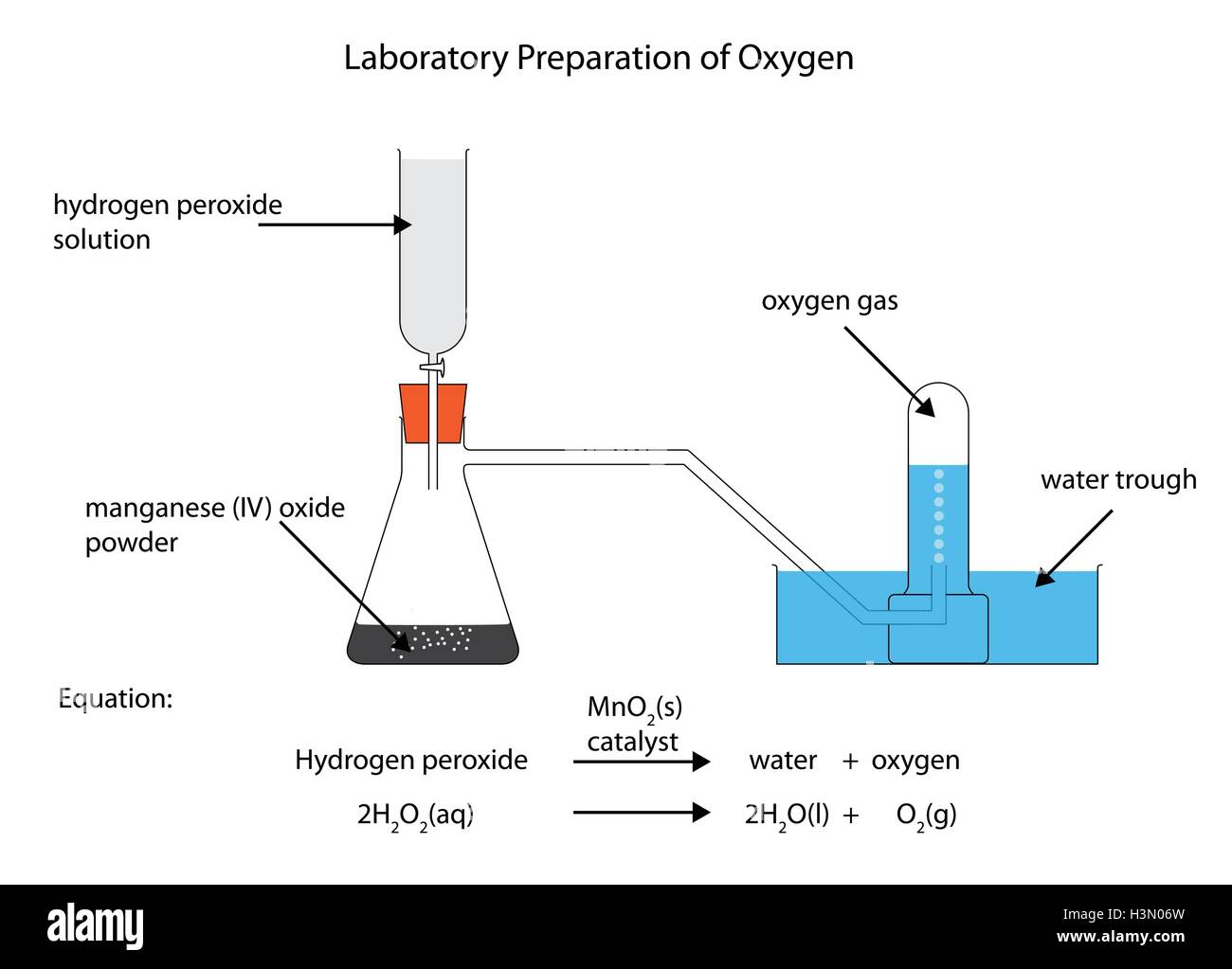
Full labelled diagram of the laboratory preparation of oxygen from hydrogen peroxide and manganese (IV) oxide Stock Vector Image & Art - Alamy

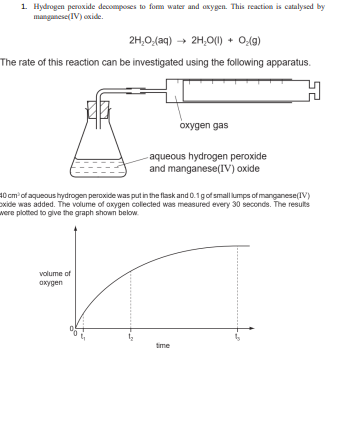
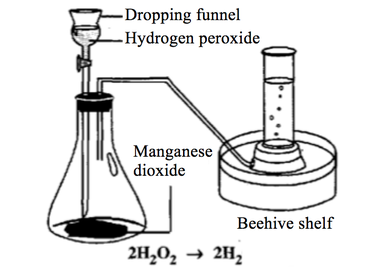
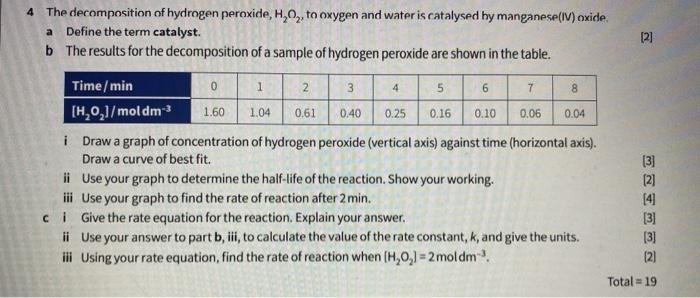
To Investigate the Effect of Manganese IV Oxide on the rate of decomposition of Hydrogen Peroxide - GCSE Science - Marked by Teachers.com
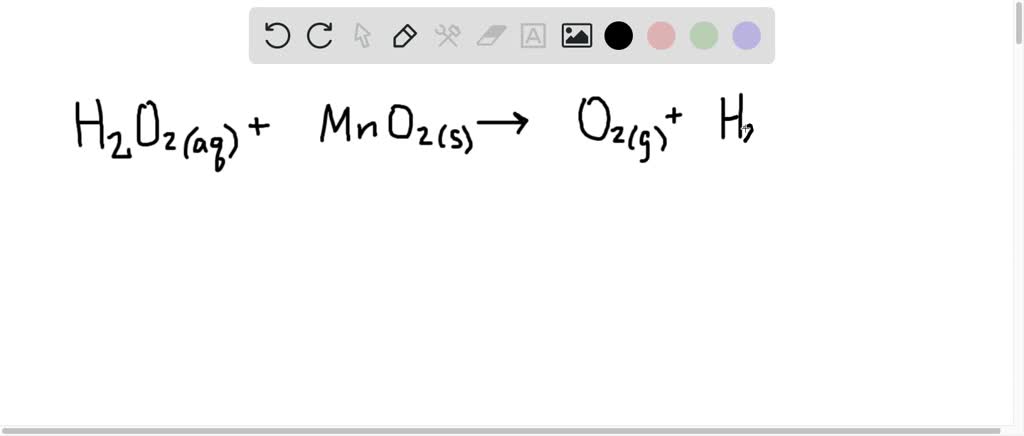
SOLVED:A common demonstration in chemistry courses involves adding a tiny speck of manganese(IV) oxide to a concentrated hydrogen peroxide, H2 O2, solution. Hydrogen peroxide is unstable, and it decomposes quite spectacularly under

inorganic chemistry - Reaction intermediates of MnO2 catalyzed H2O2 decomposition reaction - Chemistry Stack Exchange

Permanganate Reduction by Hydrogen Peroxide: Formation of Reactive Manganese Species and Superoxide and Enhanced Micropollutant Abatement | ACS ES&T Engineering
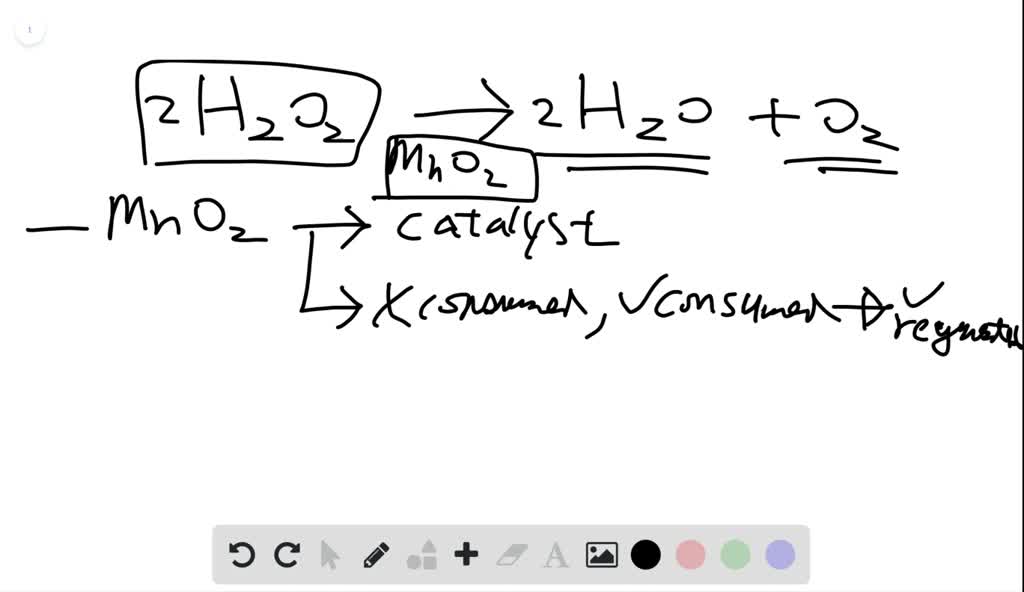
SOLVED: What happened when the manganese (IV) oxide; MnOz was added to the peroxide solution? Why did this happen? The reaction became faster because MnOz is very reactive The reaction became faster
The curves shown below were obtained when two equal volumes of hydrogen peroxide of the same - Tutorke
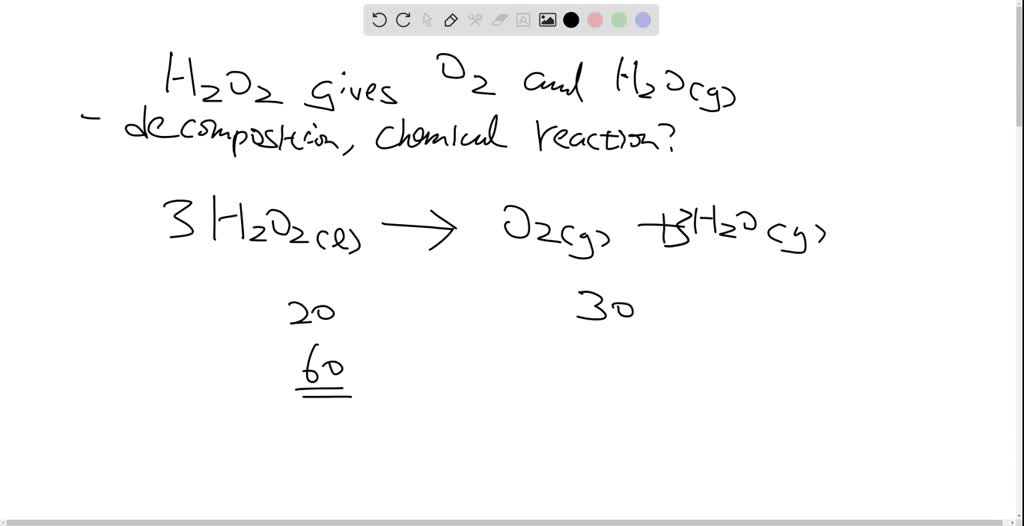
SOLVED: 1.) Manganese (IV) Oxide acts as a catalyst in this reaction, which means it is not used up and does not appear in the products.Write out the balanced equation for the