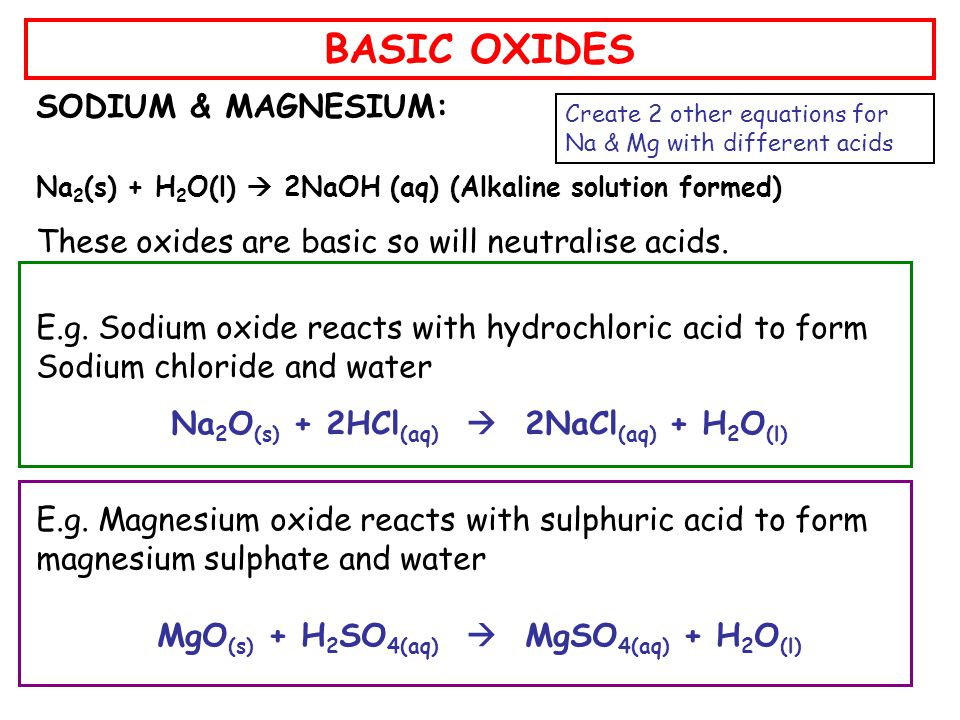
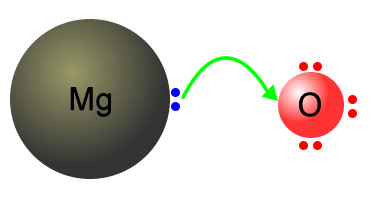
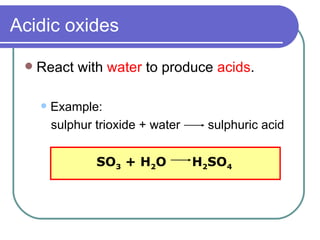
Question Video: Identifying the pH of Different Oxides by Changing the Color of the Universe Indicators in Their Solutions | Nagwa
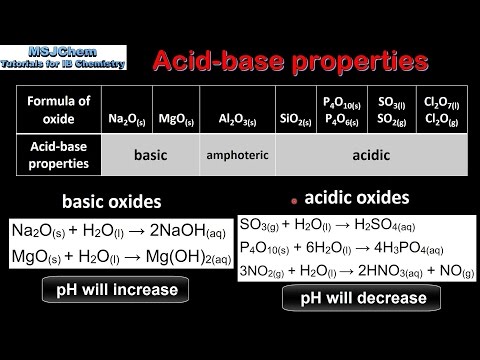
SOLVED: which of the following is acidic in natureSodium Hydroxide magnesium oxide Silver oxide Sulphur dioxide

Question Video: Determining the Color of Litmus Solution When Added to the Solution Made from Dissolving Magnesium Oxide in Water | Nagwa
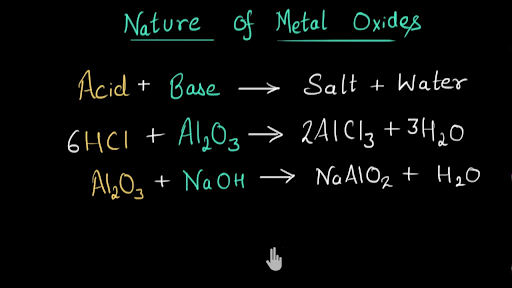
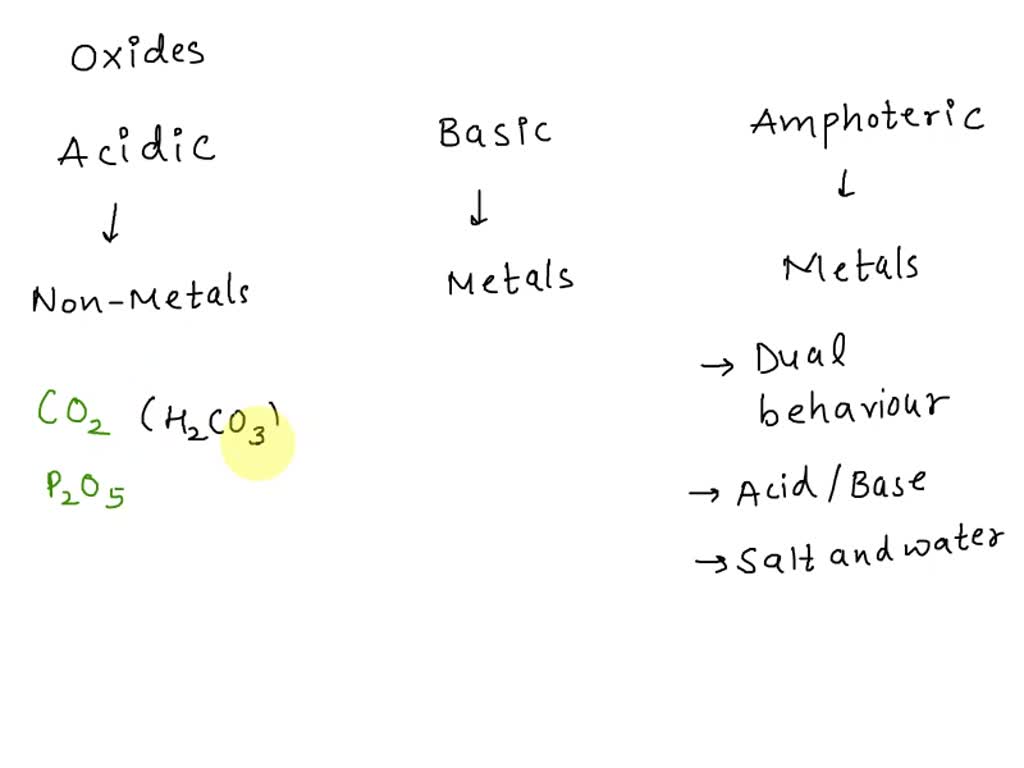
![Choose the most appropriate answer from the following list of oxides which fit the descriptions. Each answer may be used only once: [SO2, SiO2, Al2O3, MgO, CO, Na2O] (i) A basic oxide(ii) Choose the most appropriate answer from the following list of oxides which fit the descriptions. Each answer may be used only once: [SO2, SiO2, Al2O3, MgO, CO, Na2O] (i) A basic oxide(ii)](https://d1hhj0t1vdqi7c.cloudfront.net/v1/MzdncHQ0MGppZGc=/sd/)
Choose the most appropriate answer from the following list of oxides which fit the descriptions. Each answer may be used only once: [SO2, SiO2, Al2O3, MgO, CO, Na2O] (i) A basic oxide(ii)