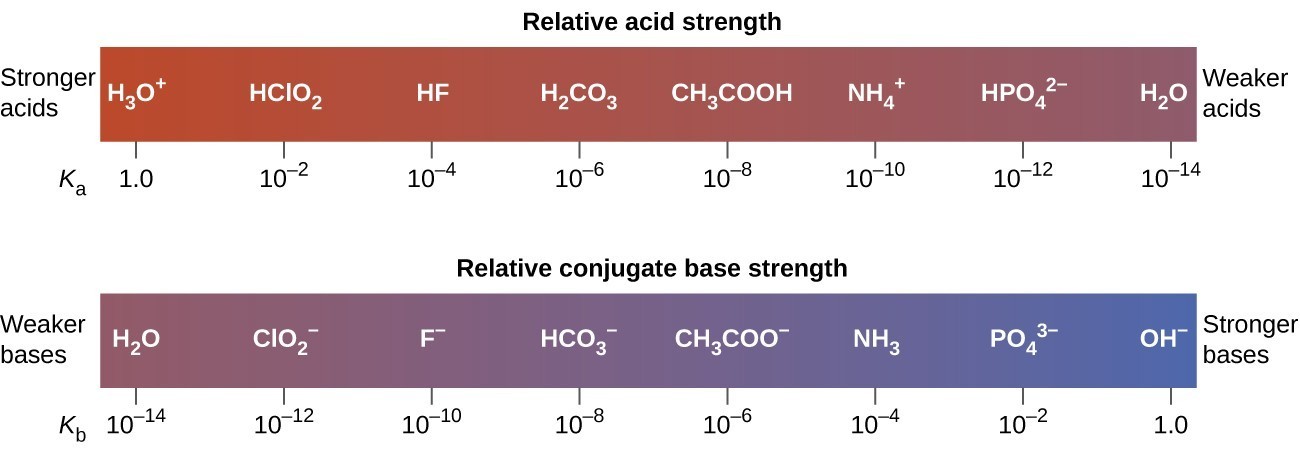
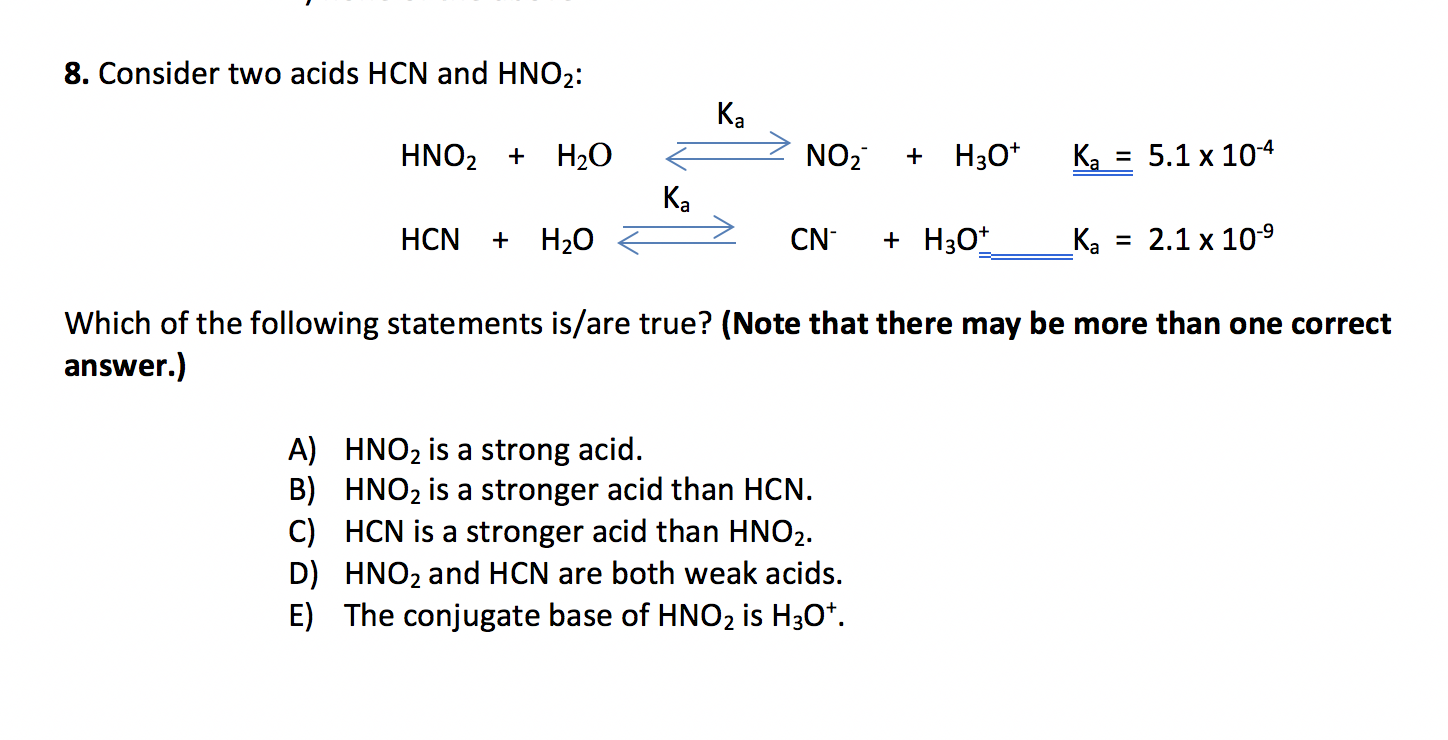
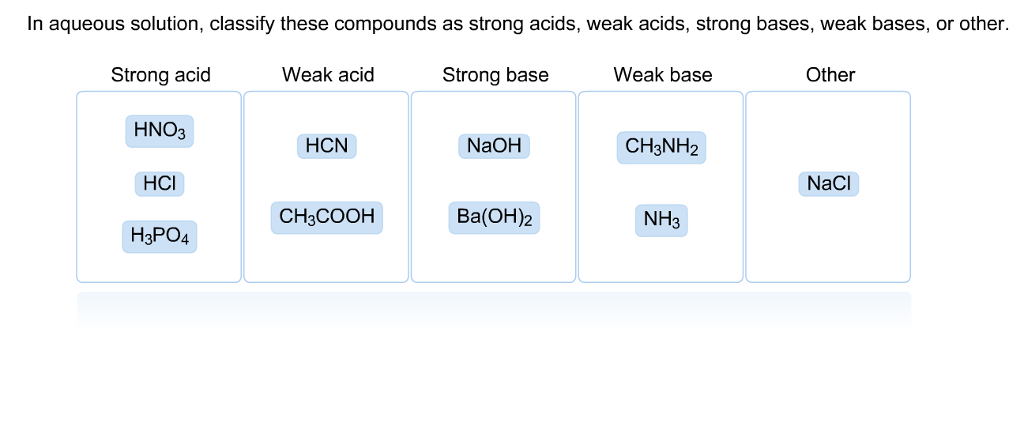
SOLVED: HCI (aq) is strong acid, which means that HCI is stronger acid that H;O . HF and HCN are weak acids, and HF is stronger than HCN. Rank the basic strength
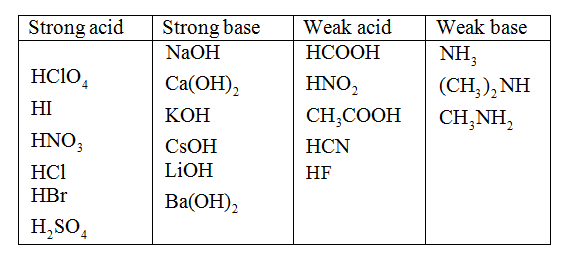
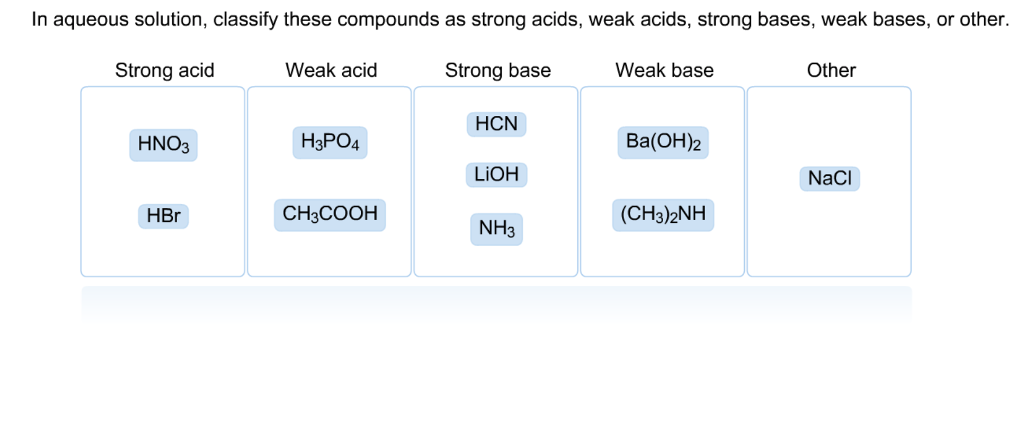
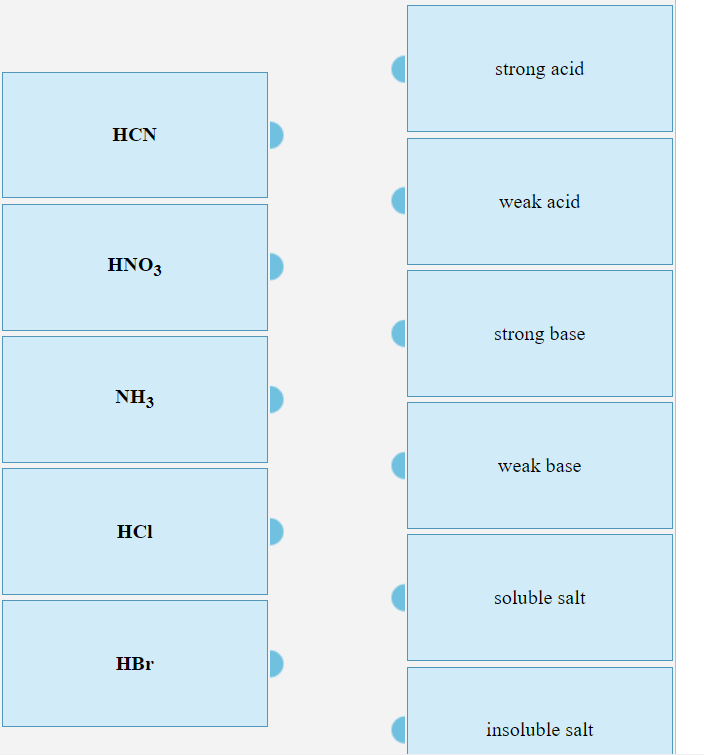
Classify each substance as a strong acid, strong base, weak acid, or weak base - Home Work Help - Learn CBSE Forum
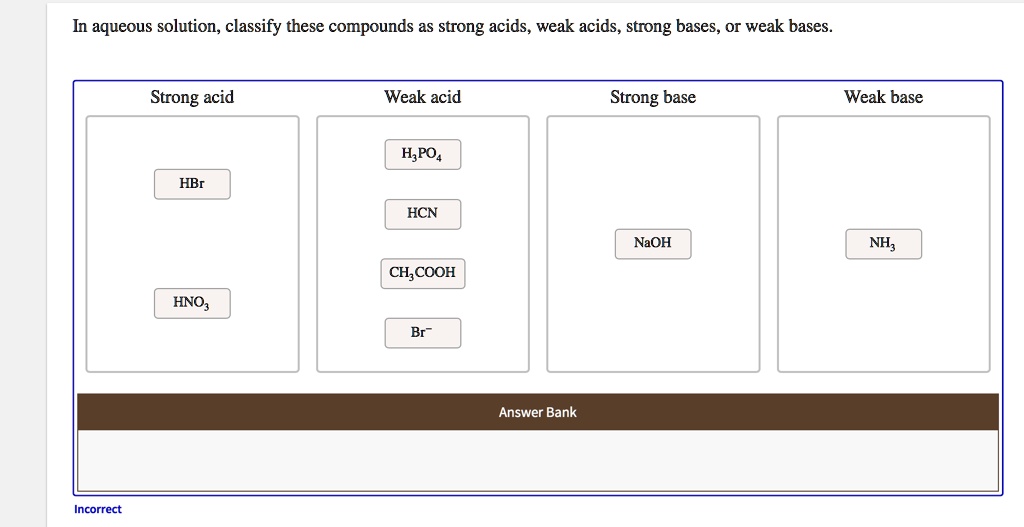
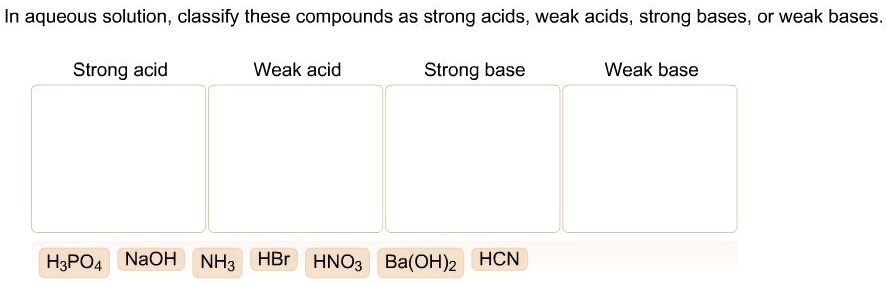
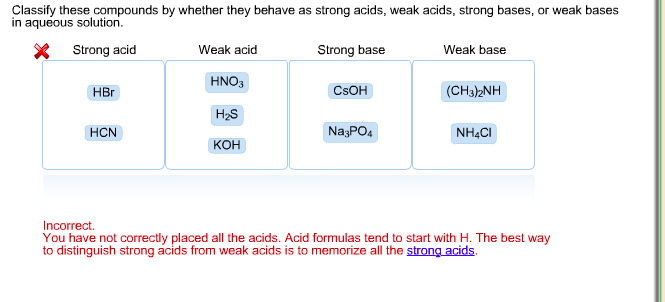
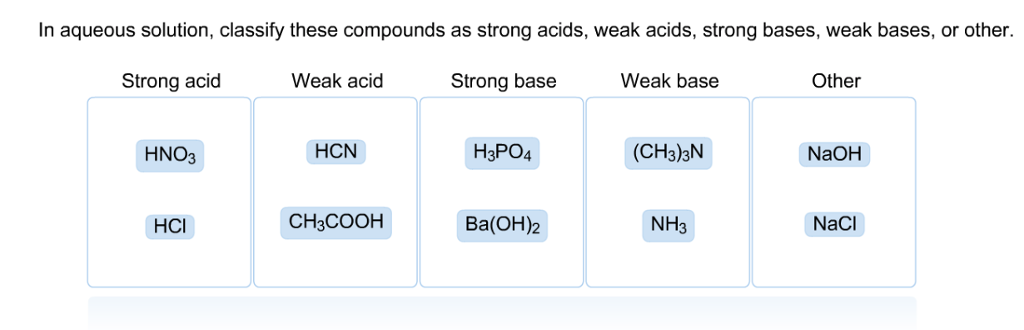
SOLVED: In aqueous solution, classify these compounds a5 strong acids, weak acids, strong bases, Or weak bases Strong acid Weak acid Strong base Weak base H;POA HBr HCN NaOH CH,COOH HNO; Br "

HCN is a weak acid ( Ka = 6.2 × 10^-10 ) ,NH4OH is a weak base ( Kb = 1.8 × 10^-5 ) . A 1.00 M solution of NH4CN would be:

Identify the stronger acid in each pair: Part A: NH^+4 or H3O^+ Part B: H2SO4 or HCN Part C: H2O or H2CO3 | Homework.Study.com

Hydrogen cyanide (HCN)- Lewis acid Structure, Molecular mass, Physical and Chemical Properties, Uses with FAQs of Hydrogen Cyanide.