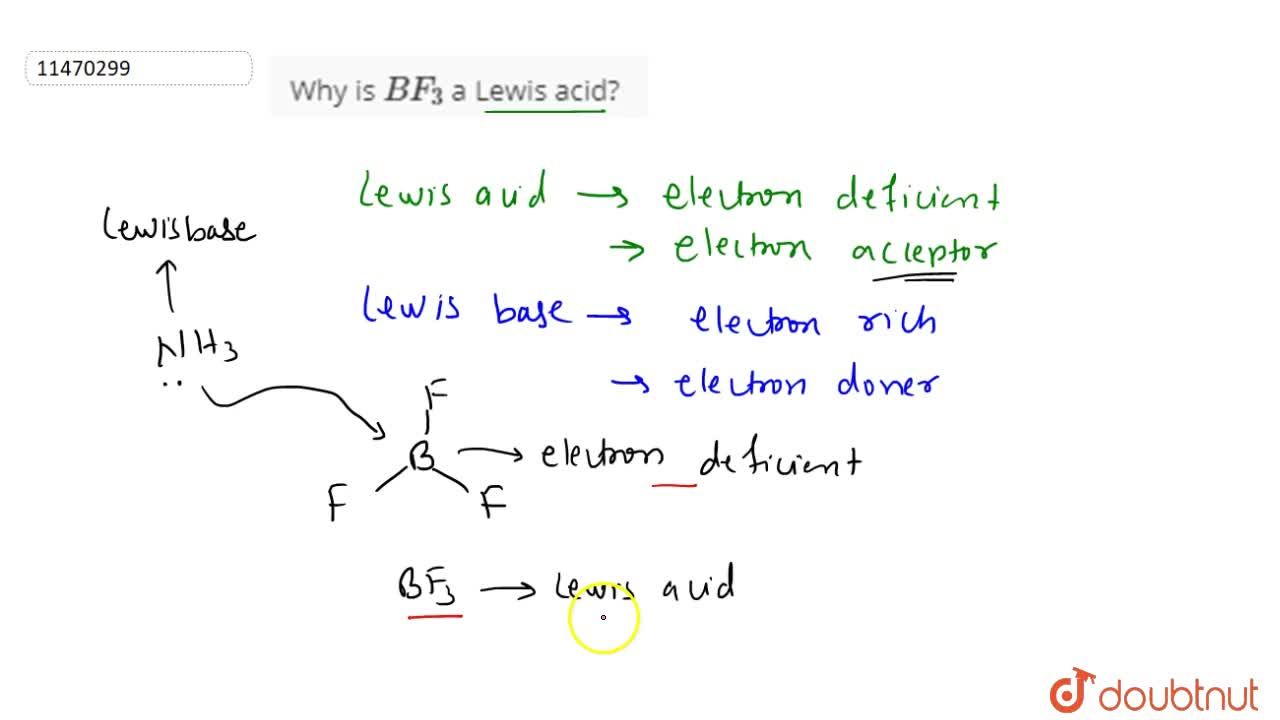
Explain : (A) BF3 is a Lewis acid, (B) NH3 is a Lewis base. - Sarthaks eConnect | Largest Online Education Community

Question Video: Identifying the Species That Is Not a Lewis Acid in a Set of Chemical Formulas | Nagwa

Draw structural formulas to represent the reaction between a Lewis acidand a Lewis base and identify the nature of the bond formed in the product

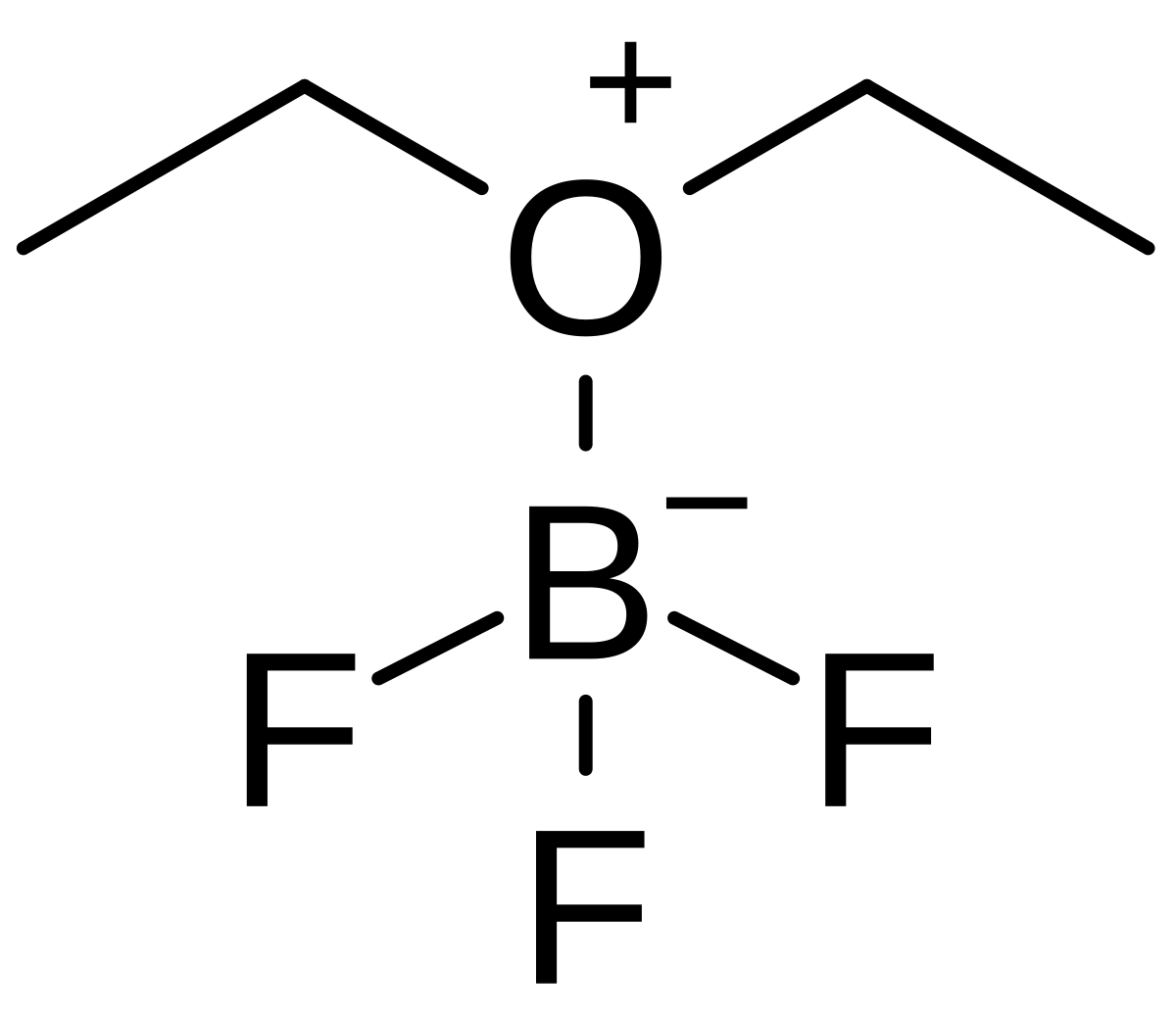
Draw the lewis structure of the 1:1 adduct that forms in the lewis acid-base reaction between boron - Brainly.com
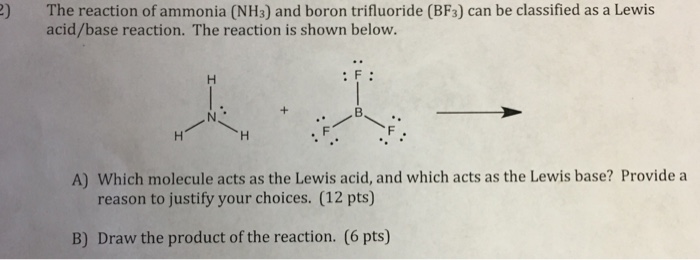
Why does boron trifluoride (BF3) act as a Lewis base and ammonia (NH3) acts as a Lewis acid? - Quora
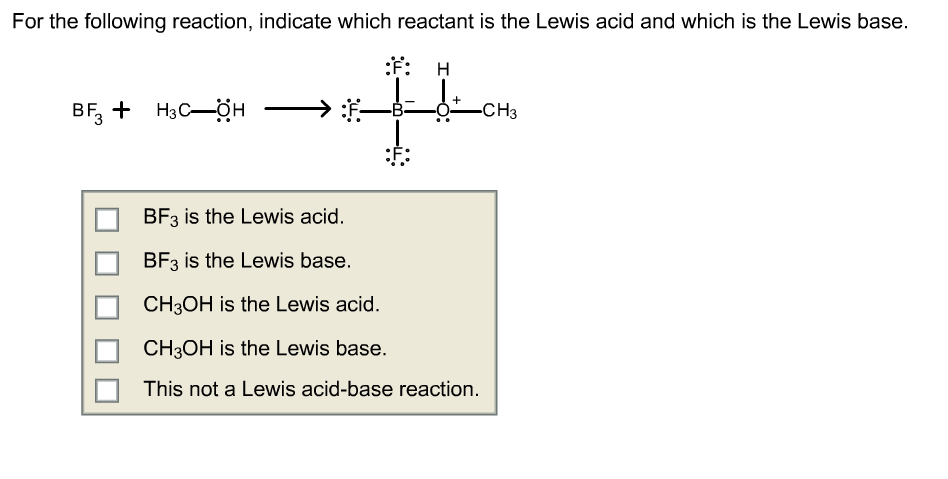
SOLVED: Identify the Lewis Acid in the following reaction: (CH3)2o BF3 (CH3)OBF3 (CH3)OBF3 (CH3h)20 None This is not a Lewis Acid-Base reaction. BF3

Why BF3 acts as Lewis Acid | Boron trifluoride acts as a Lewis Acid why | Lewis Acid and Base Theory - YouTube

Boron trifluoride, BF3, and ammonia, NH3, react to produce BF3 : NH3. A coordinate covalent bond is formed between the boron atom on BF3 and the nitrogen atom on NH3. Write the