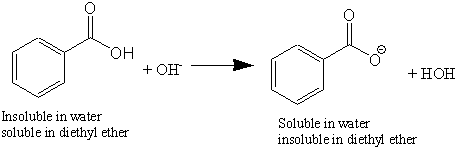
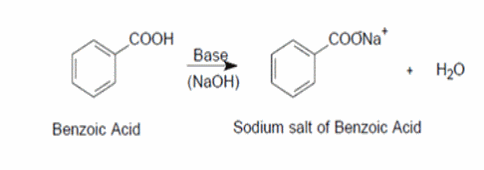
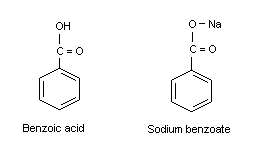
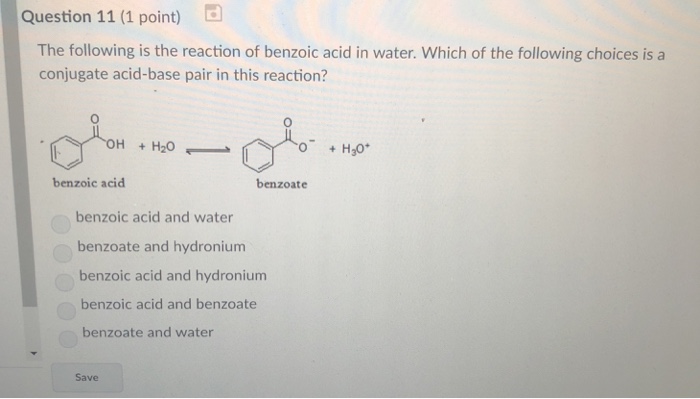
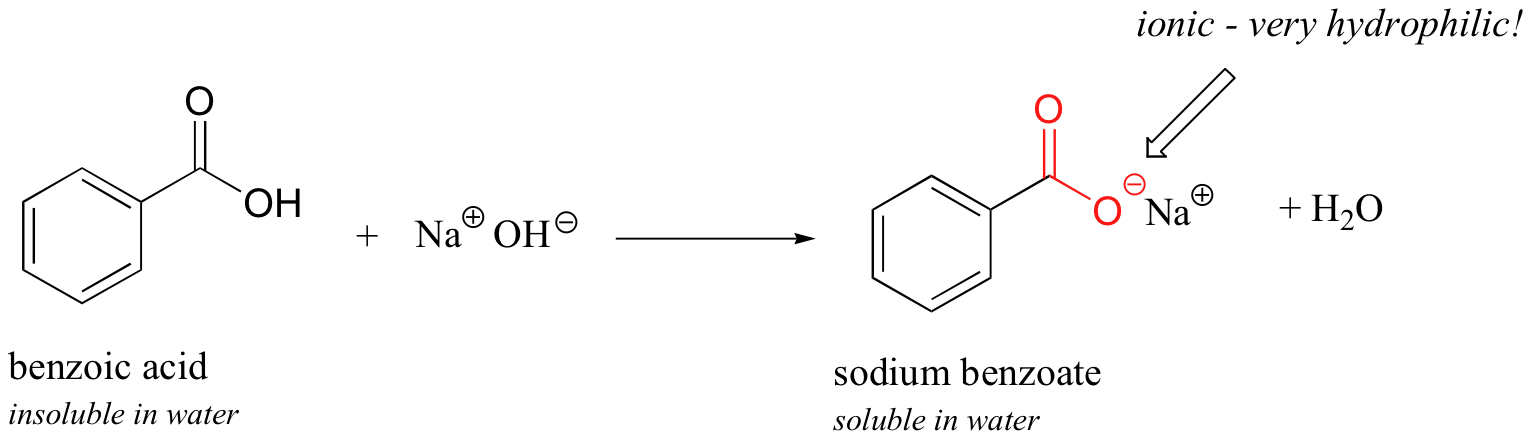
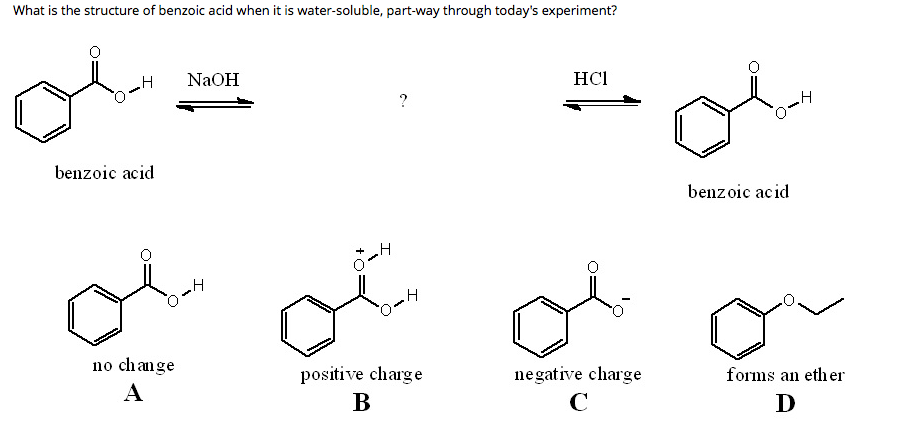
Explain why the following statement is incorrect: Benzoic acid dissolved in water upon the addition of a saturated solution of sodium bicarbonate. | Homework.Study.com

Which of these is the most acidic compound; Benzoic acid, ethyl 4-amino benzoate, 1,4-dimethoxybenzene? Provide a mechanism for its conversion into water soluble compound. Include a reasonable base and show all electron

Solved) - Will sodium benzoate be more soluble in water than benzoic acid.... (1 Answer) | Transtutors

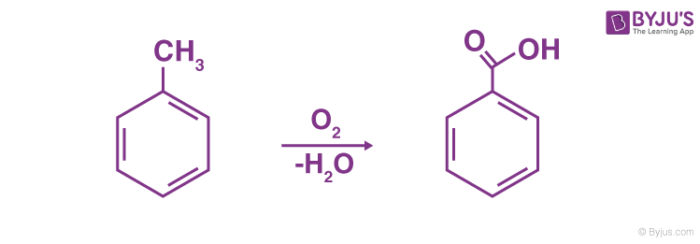
Physical chemical properties benzoic acid aromatic acids benzamide benzonitrile benzoyl chloride electrophilic substitution nitration halogenation reaction with alkalis carbonates benzonitrile benzamide advanced A level organic chemistry revision notes ...

Continuous Cocrystallization of Benzoic Acid and Isonicotinamide by Mixing-Induced Supersaturation: Exploring Opportunities between Reactive and Antisolvent Crystallization Concepts | Crystal Growth & Design