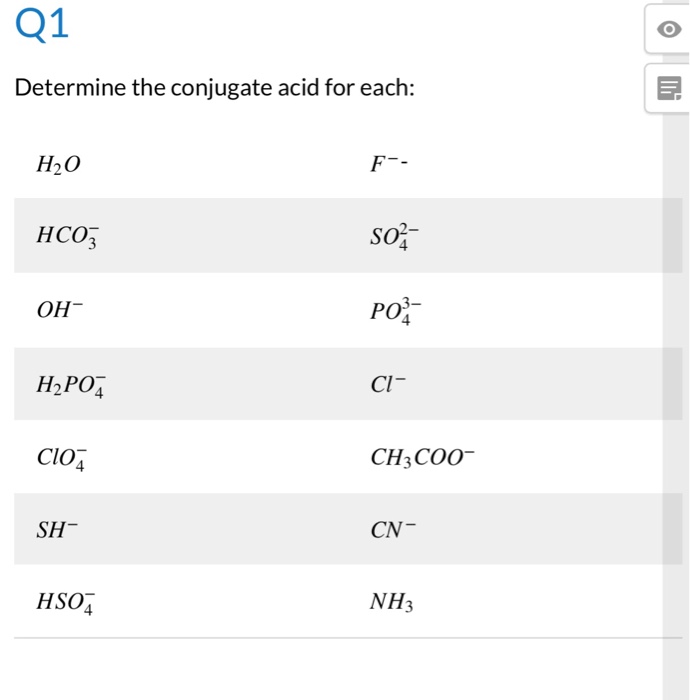
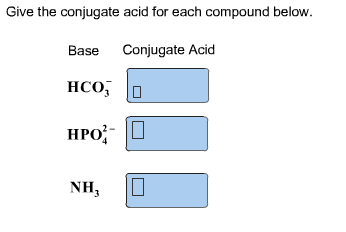
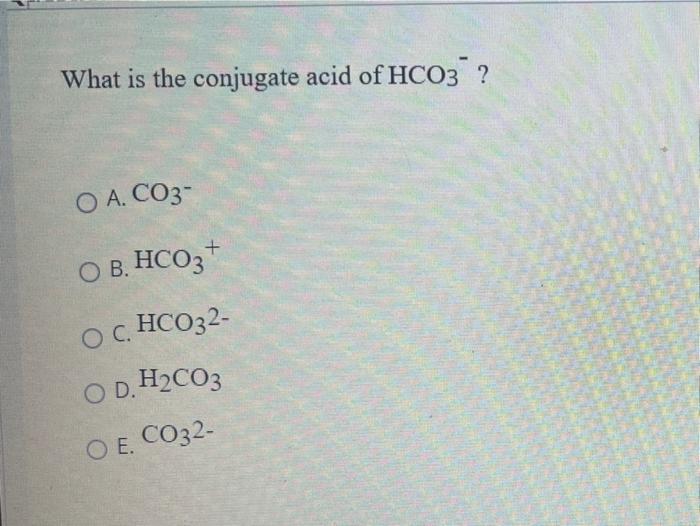The species: H2O, HCO3^-, HSO4^- and NH3 can act both as Bronsted acids and bases. For each case give the corresponding conjugate acid and base.

Practice problems Identify the acid, base, conjugate acid, conjugate base, and conjugate acid-base pairs: HC2H3O2(aq) + H2O(l) C2H3O2–(aq) + H3O+(aq) - ppt video online download
Free Solution] For the reaction H2CO3(aq)+CN^-(aq)⇌ HCN(aq)+HCO3^-(aq) label each species as an acid or a...

What is the conjugate base of HCO3−? Express your answer as a chemical formula - Home Work Help - Learn CBSE Forum

SOLVED: The hydrogen carbonate ion, HCO3 , has both a conjugate acid and a conjugate base. These are; respectively: CO;? HCO; Hzot, OH HzCO;, CO; HCO;, HCO; HCO; HCO;
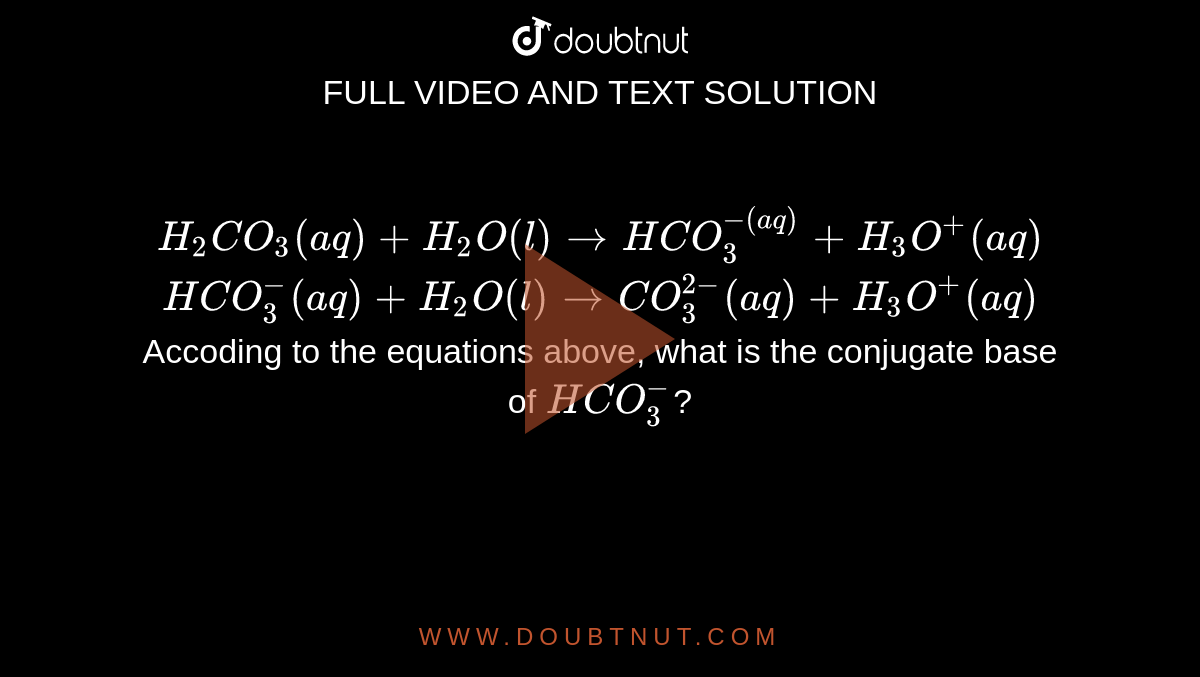
H2CO3(aq)+H2O(l)toHCO3^-(aq) + H3O^+(aq) HCO3^(-)(aq) + H2O(l)to CO3^(2-)(aq)+H3O^(+)(aq) Accoding to the equations above, what is the conjugate base of HCO3^-?
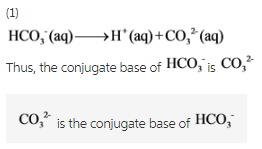


![ANSWERED] HCO3 + H₂→ H₂CO3 + OH In the equation abov... - Organic Chemistry ANSWERED] HCO3 + H₂→ H₂CO3 + OH In the equation abov... - Organic Chemistry](https://media.kunduz.com/media/sug-question/raw/44878390-1658615733.1594505.jpeg)