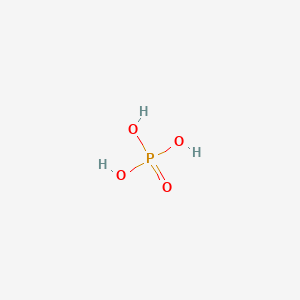
![Orthophosphoric Acid (H3PO4) [Phosphoric Acid] - Structure, Formula, Synthesis, Properties, Uses and FAQs of Orthophosphoric acid. Orthophosphoric Acid (H3PO4) [Phosphoric Acid] - Structure, Formula, Synthesis, Properties, Uses and FAQs of Orthophosphoric acid.](https://cdn1.byjus.com/wp-content/uploads/2018/11/phosphoric-acid-structure.png)
Orthophosphoric Acid (H3PO4) [Phosphoric Acid] - Structure, Formula, Synthesis, Properties, Uses and FAQs of Orthophosphoric acid.
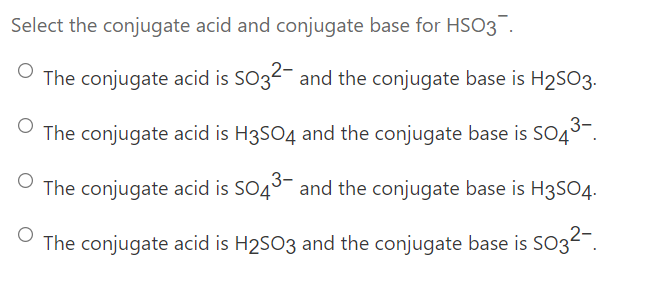
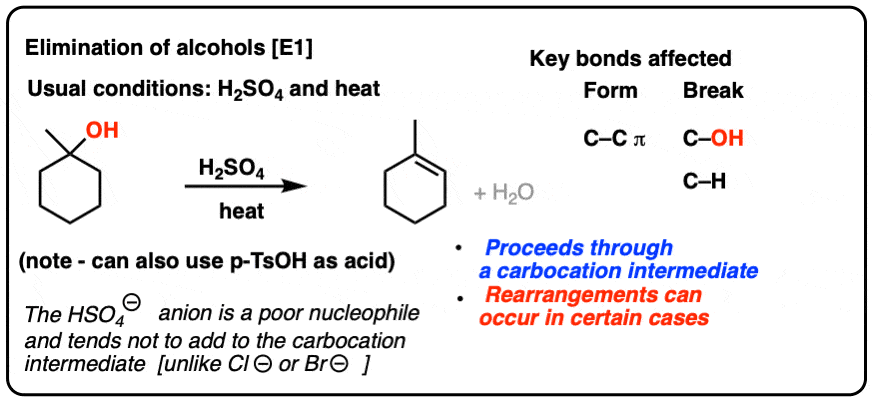
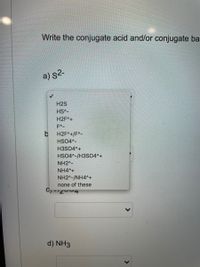
Conjugate Acid-Base Pairs Ordered by Strength Acids Bases [strong] [weak] HClO4 ClO4 H2SO4 HSO4 HCl HNO3 NO3 H3O+ H2O H2C2O4 (ox

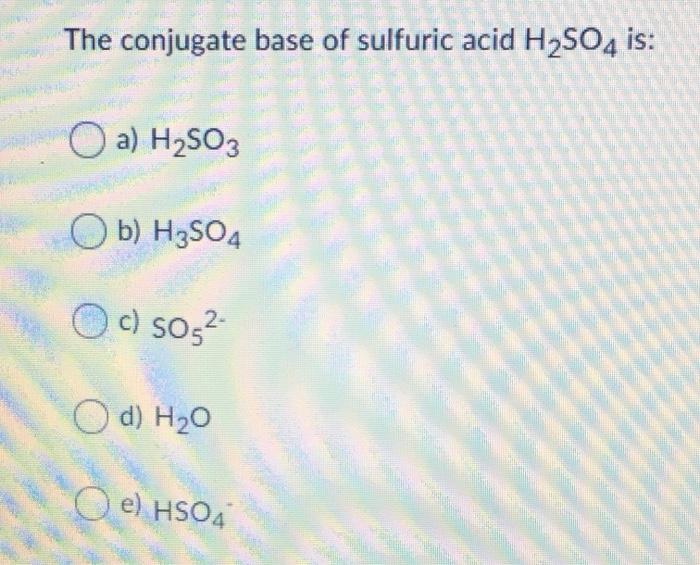
Identify the following reaction, label the acid, conjugate acid, base , and conjugate base: H2SO4 + H2O = HSO4 + H3O+ | Wyzant Ask An Expert
For ortho phosphoric acid, H3PO4(aq)+H2O(aq) to H3O^(+)(aq)+H2PO4^(-)(aq),K(a1) H2PO4^-(aq)+H2O(aq) to H3O^(+)(aq)+H2PO4^(-)(aq),K(a2) HPO4^(2-)(aq)+H2O(aq) to H3O^(+)(aq)+PO3^(4)(aq),K(a3) The correct order of Ka values is :