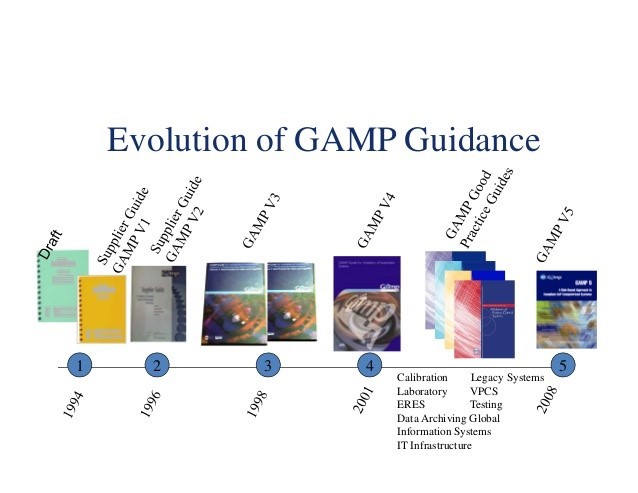
GAMP RDI Good Practice Guide: Data Integrity by Design | ISPE | International Society for Pharmaceutical Engineering
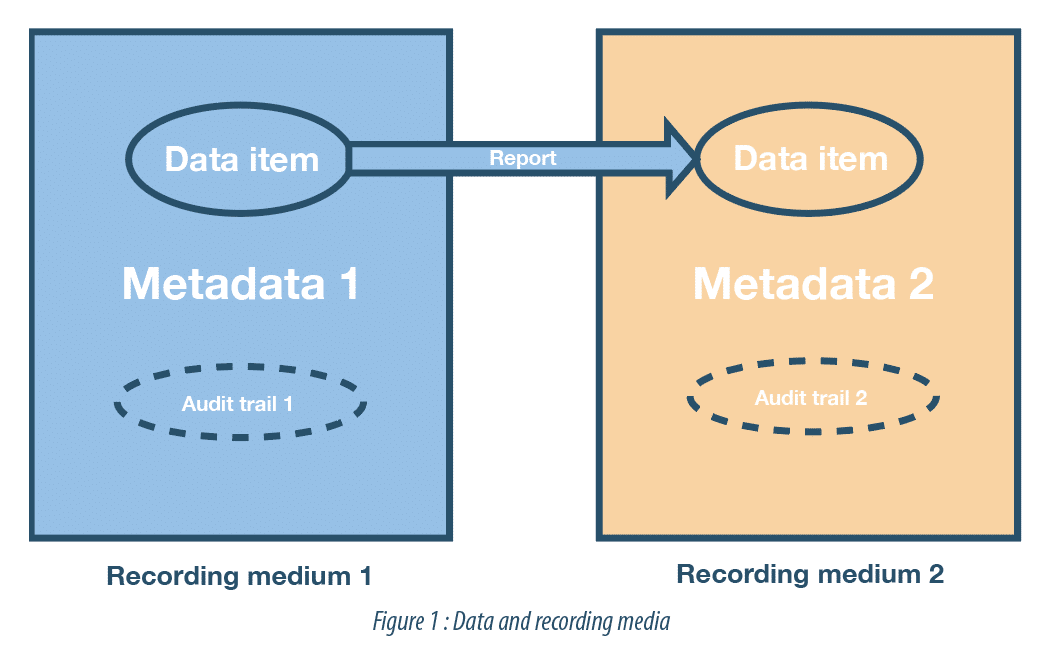
Backup, replication and archiving… What measures to take to preserve the integrity of your data? - A3P - Pharmaceutical & Biotechnology Industry
Good laboratory practice (GLP). Guidelines for the archiving of electronic raw data in a GLP environment<link href='#fn1&