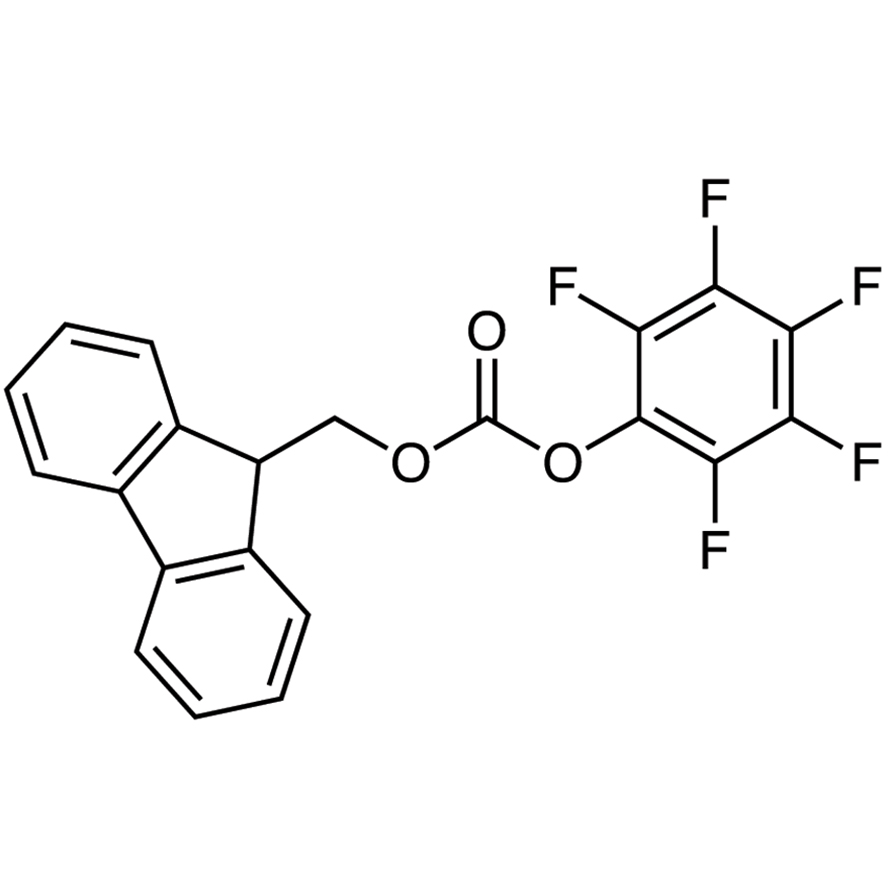
EP0410263B1 - Cationic photoinitiated polymerisation process and the preparation of relief motifs or photoimages using sulfonium salts - Google Patents
Use of Phthaloyl Protecting Group for the Automated Synthesis of 3'-[(Hydroxypropyl)amino] and 3'-[(Hydroxypropyltriglyc
![The 1,1-Dioxobenzo[b]thiophene-2-ylmethyloxycarbonyl (Bsmoc) Amino-Protecting Group | The Journal of Organic Chemistry The 1,1-Dioxobenzo[b]thiophene-2-ylmethyloxycarbonyl (Bsmoc) Amino-Protecting Group | The Journal of Organic Chemistry](https://pubs.acs.org/cms/10.1021/jo982140l/asset/images/medium/jo982140ln00001.gif)
The 1,1-Dioxobenzo[b]thiophene-2-ylmethyloxycarbonyl (Bsmoc) Amino-Protecting Group | The Journal of Organic Chemistry
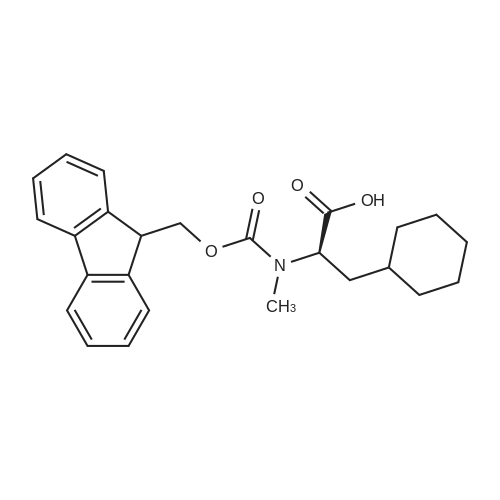
148983-03-3|(S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)(methyl)amino)-3-cyclohexylpropanoic acid| Ambeed

Synthetic Procedure for N-Fmoc Amino Acyl-N-Sulfanylethylaniline Linker as Crypto-Peptide Thioester Precursor with Application to Native Chemical Ligation | The Journal of Organic Chemistry

Design and Molecular dynamic Investigations of 7,8-Dihydroxyflavone Derivatives as Potential Neuroprotective Agents Against Alpha-synuclein | Scientific Reports
![PDF) Isocyanates of N α -[(9-Fluorenylmethyl)oxy]carbonyl Amino Acids: Synthesis, Isolation, Characterization, and Application to the Efficient Synthesis of Urea Peptidomimetics | Vasanthakumar Ganga Ramu - Academia.edu PDF) Isocyanates of N α -[(9-Fluorenylmethyl)oxy]carbonyl Amino Acids: Synthesis, Isolation, Characterization, and Application to the Efficient Synthesis of Urea Peptidomimetics | Vasanthakumar Ganga Ramu - Academia.edu](https://0.academia-photos.com/14265805/16551552/16877989/s200_vasanthakumar.ganga_ramu.jpg)
PDF) Isocyanates of N α -[(9-Fluorenylmethyl)oxy]carbonyl Amino Acids: Synthesis, Isolation, Characterization, and Application to the Efficient Synthesis of Urea Peptidomimetics | Vasanthakumar Ganga Ramu - Academia.edu
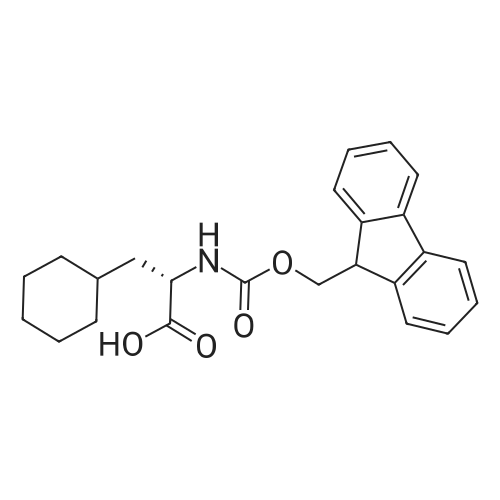
148983-03-3|(S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)(methyl)amino)-3-cyclohexylpropanoic acid| Ambeed

Synthetic Procedure for N-Fmoc Amino Acyl-N-Sulfanylethylaniline Linker as Crypto-Peptide Thioester Precursor with Application to Native Chemical Ligation | The Journal of Organic Chemistry

Complex Polyfluoride Additives in Fmoc-Amino Acid Fluoride Coupling Processes. Enhanced Reactivity and Avoidance of Stereomutation | Organic Letters
Factor IX Zutphen: a Cys18-->Arg mutation results in formation of a heterodimer with alpha 1-microglobulin and the inability to form a calcium-induced conformation. - Abstract - Europe PMC

Synthetic Procedure for N-Fmoc Amino Acyl-N-Sulfanylethylaniline Linker as Crypto-Peptide Thioester Precursor with Application to Native Chemical Ligation | The Journal of Organic Chemistry
9-Fluorenylmethyl)oxy]carbonyl (FMOC) amino acid fluorides. Convienient new peptide coupling reagents applicable to the FMOC/tert-butyl strategy for solution and solid-phase syntheses. | Journal of the American Chemical Society
148983-03-3|(S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)(methyl)amino)-3-cyclohexylpropanoic acid| Ambeed

Synthetic Procedure for N-Fmoc Amino Acyl-N-Sulfanylethylaniline Linker as Crypto-Peptide Thioester Precursor with Application to Native Chemical Ligation | The Journal of Organic Chemistry