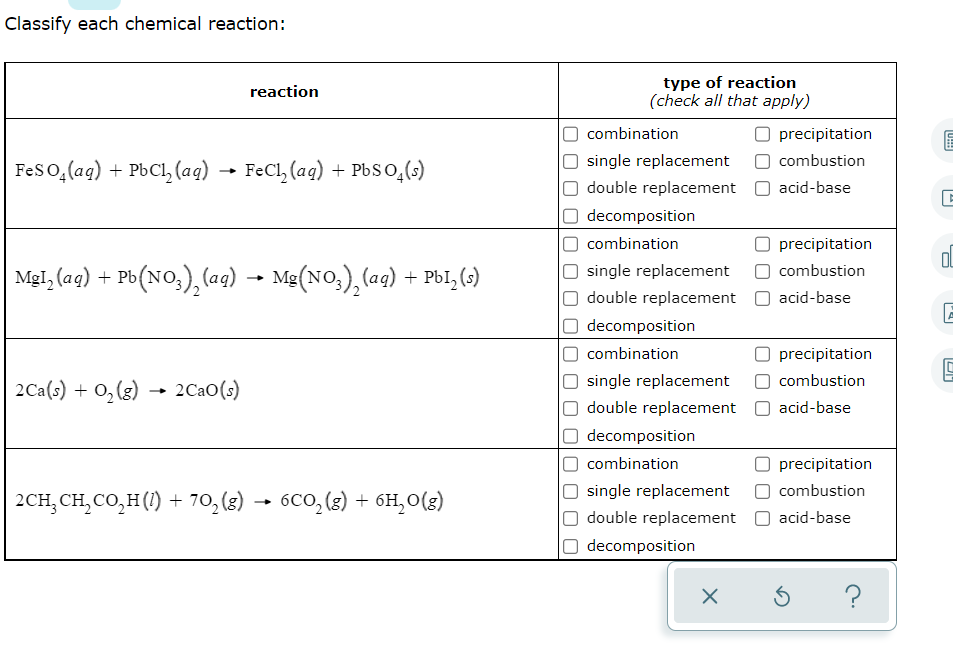
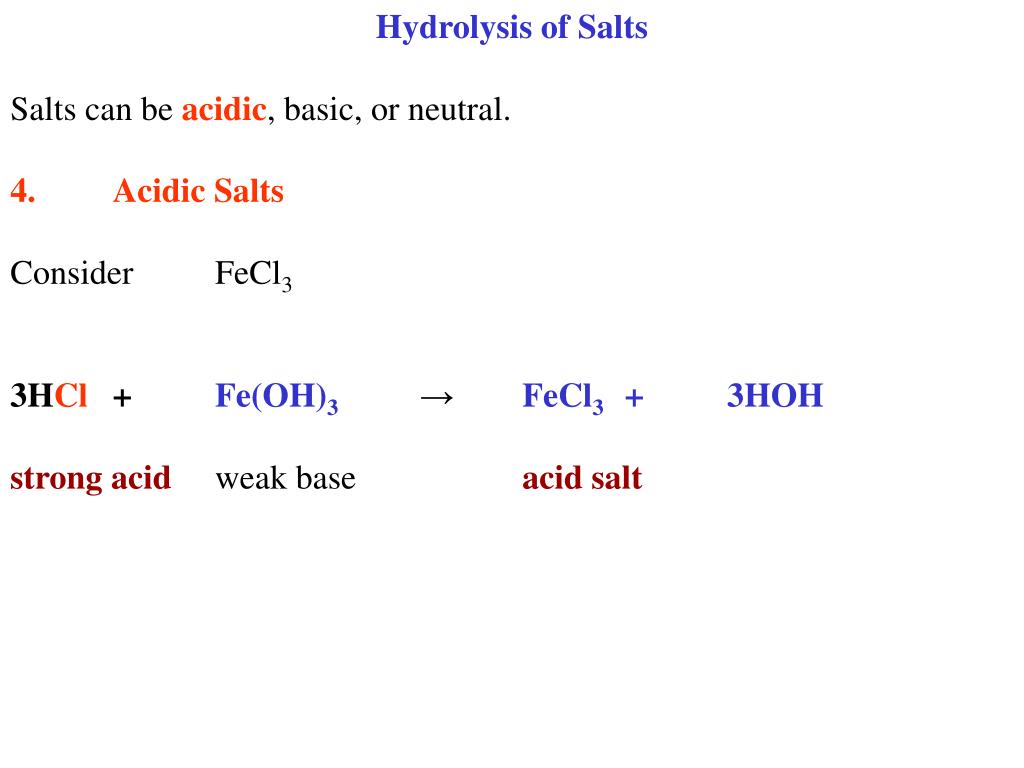
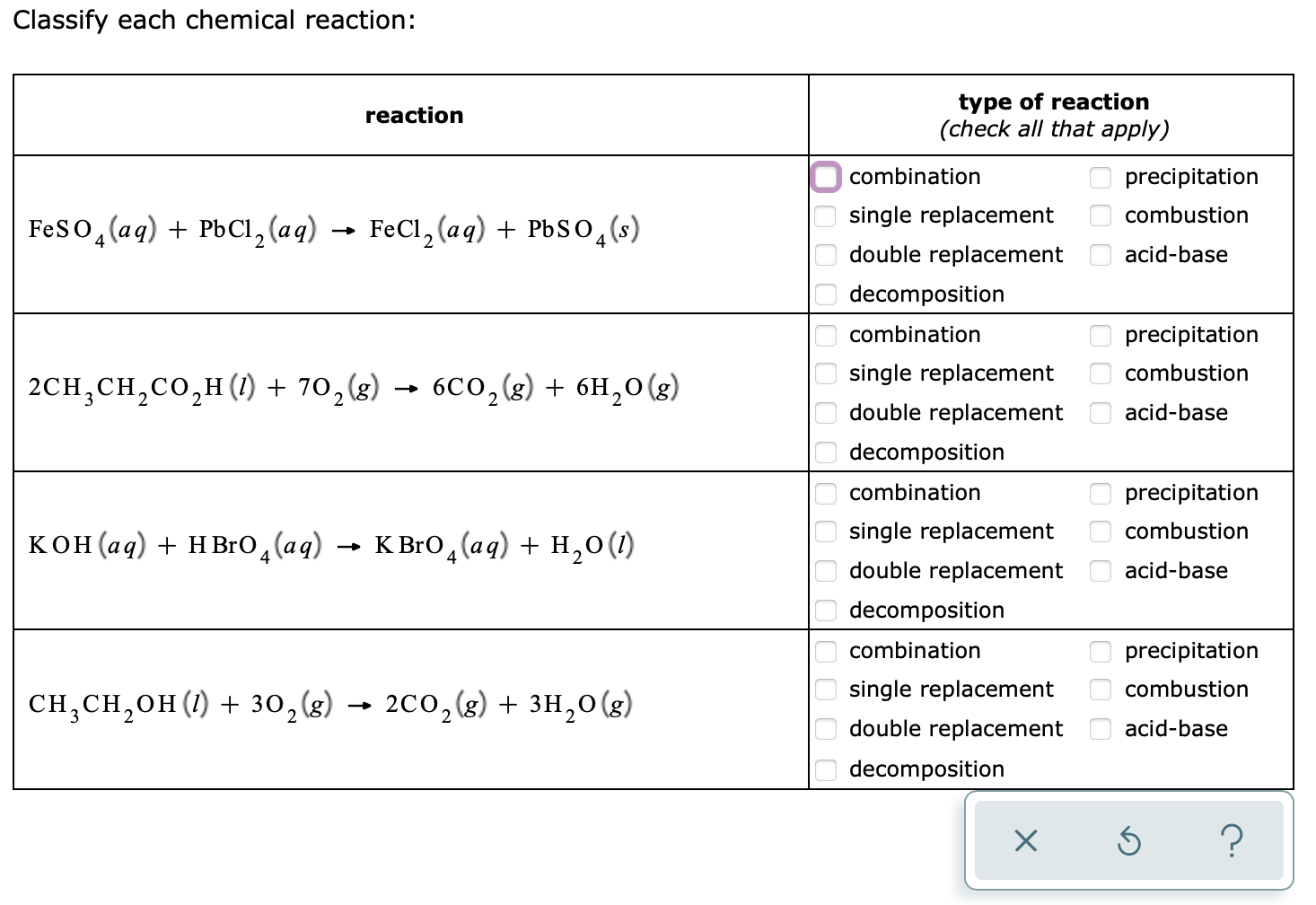
Acids and Bases Chapter 14 Acids and Bases. Acids and Bases Some Definitions Arrhenius Acid:Substance that, when dissolved in water, increases the concentration. - ppt download
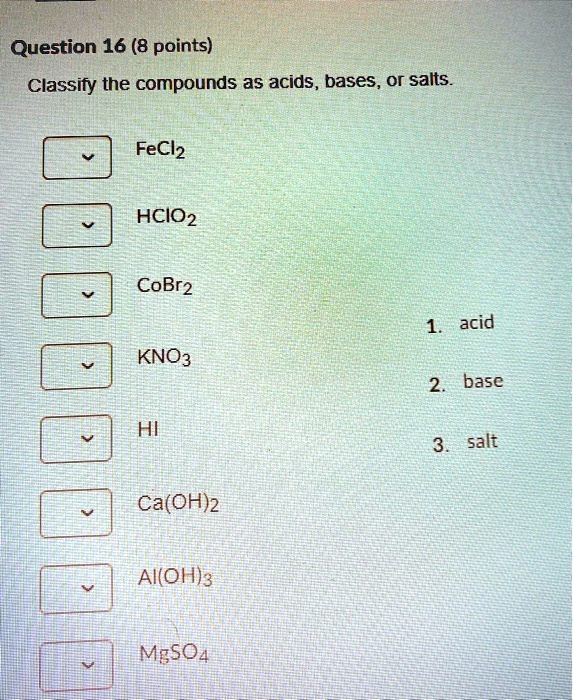
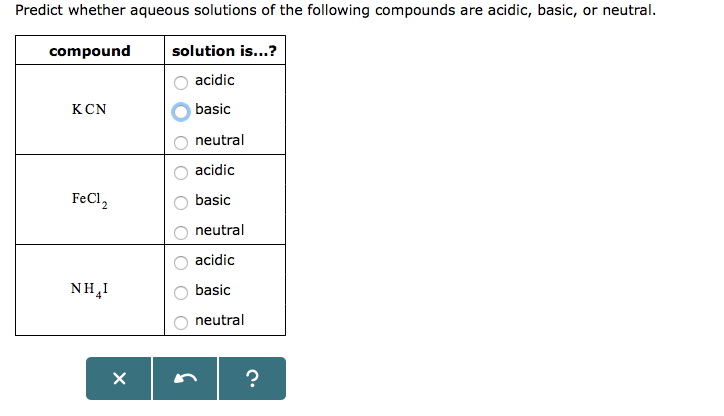
SOLVED: Question 16 (8 points) Classify the compounds as acids, bases, or salts FeCl2 HCIO2 acid KNO3 basc HI salt ca(OH)2 AICOH)3 M,5o4 CoBr2
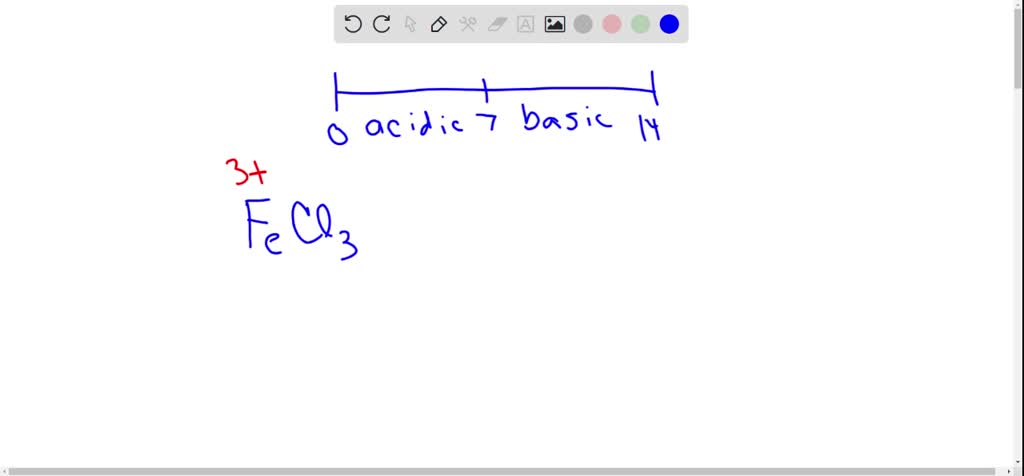
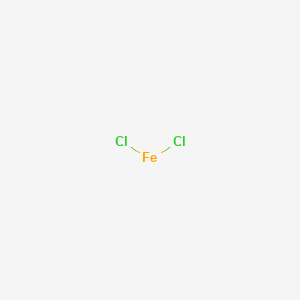
SOLVED: Metallic ions with a higher positive charge are more strongly hydrated and tend to be more acidic in solution. Comparing a 0.12 M FeCl3 solution to a 0.12 M FeCl2 solution,