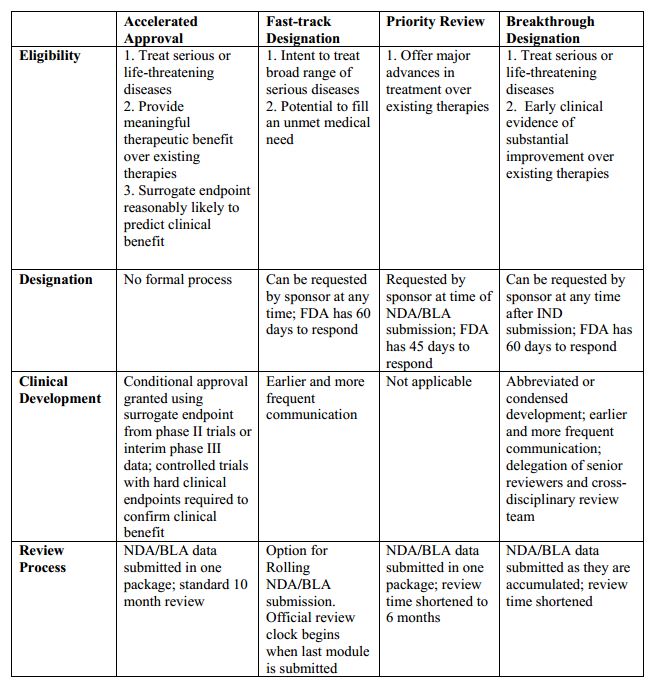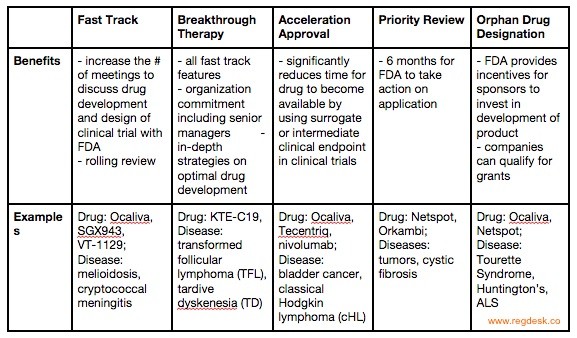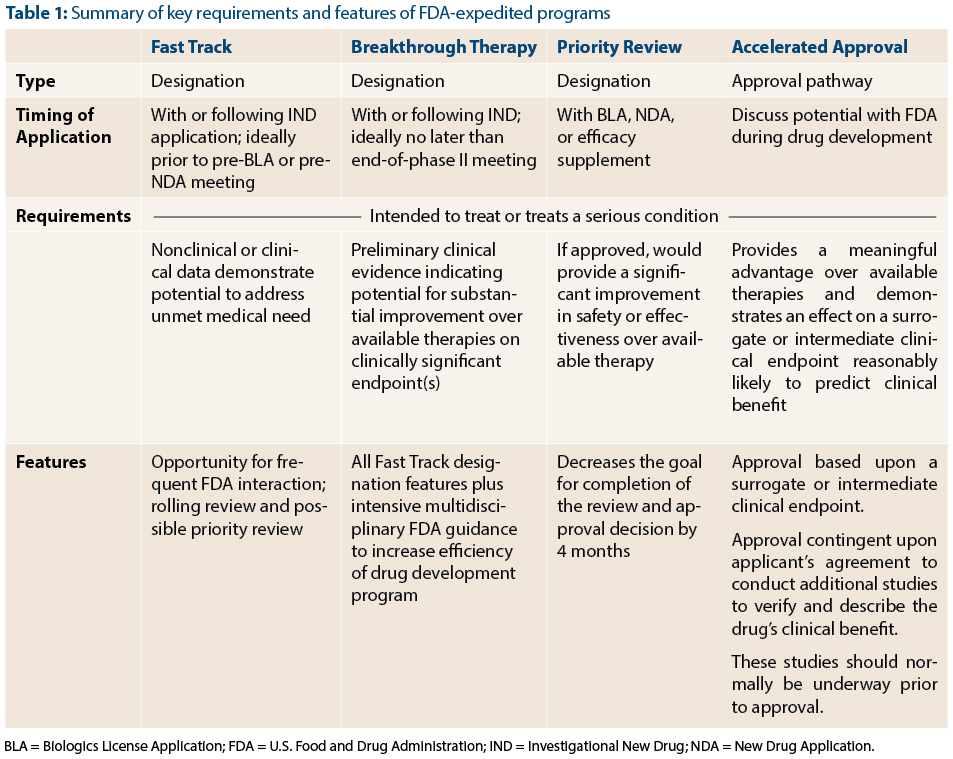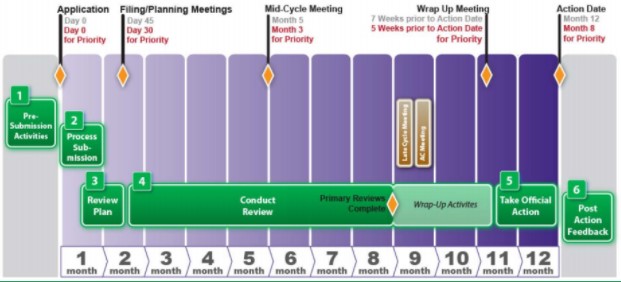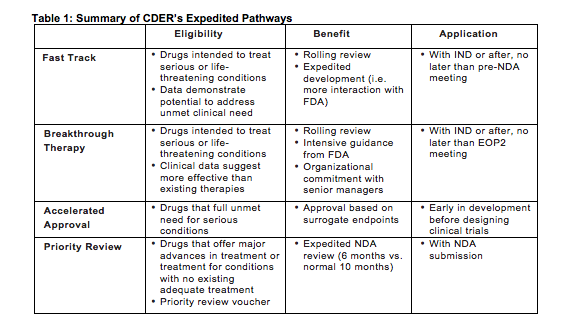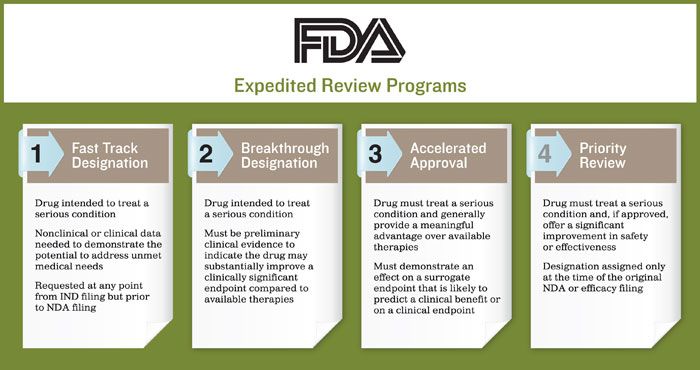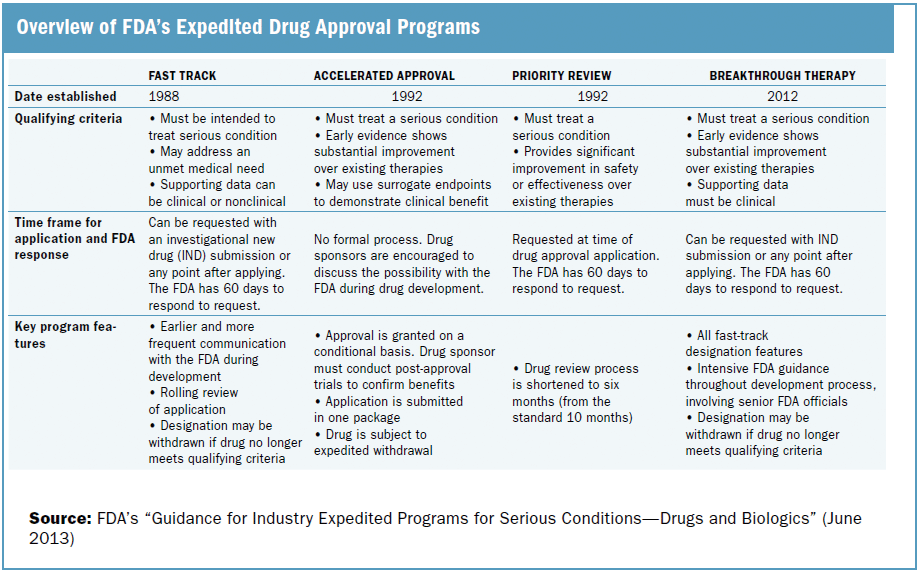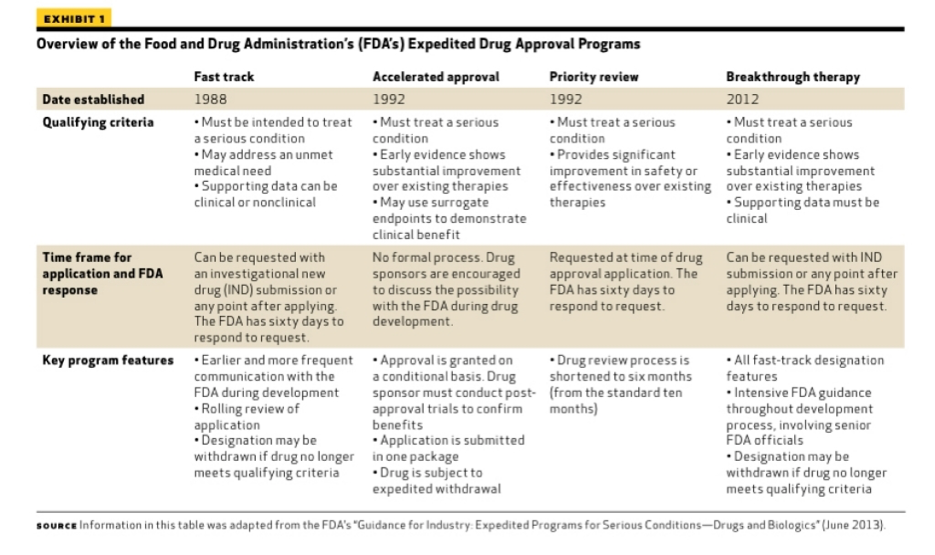
The Science Of A Biotech Valuation: How To Interpret The Value Of FDA Expedited Programs (NASDAQ:IBB) | Seeking Alpha

CorMedix eyes US FDA priority review for bloodstream infection drug Defencath | S&P Global Market Intelligence
The Need for Speed in Drug Development: A Sponsor's Guide to FDA Expedited Programs | Halloran Consulting Group