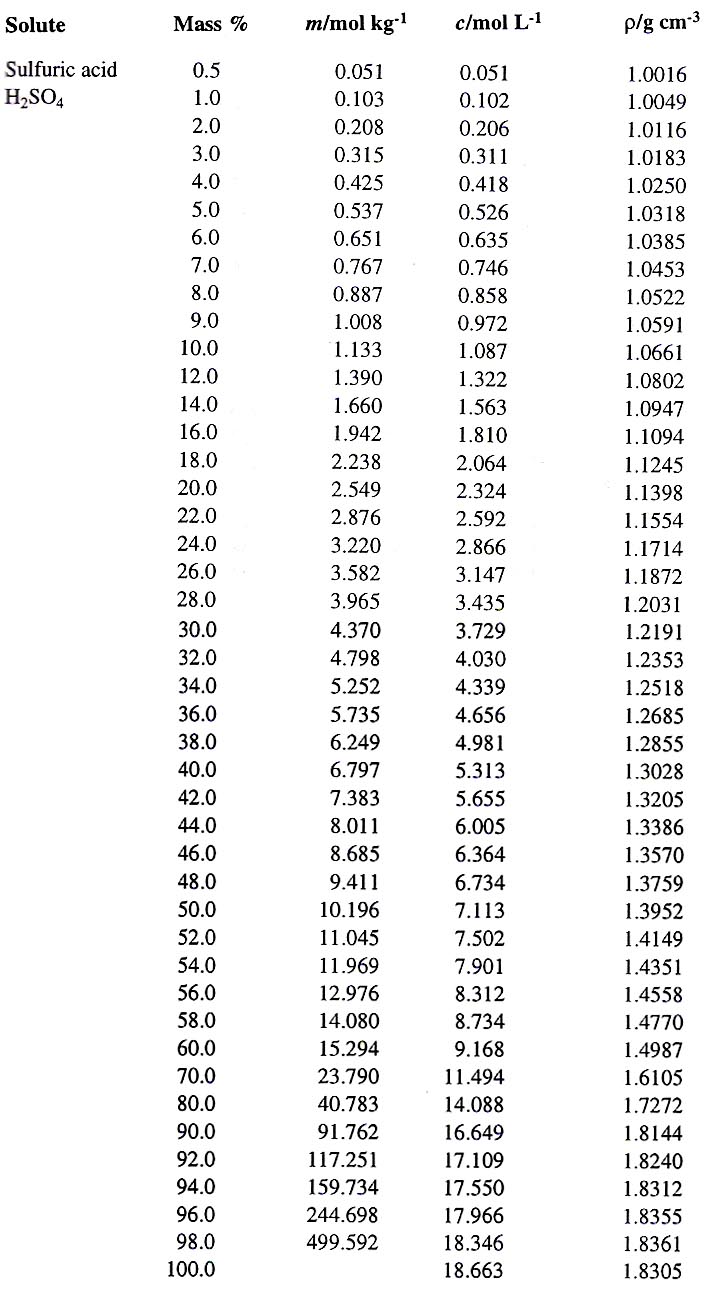
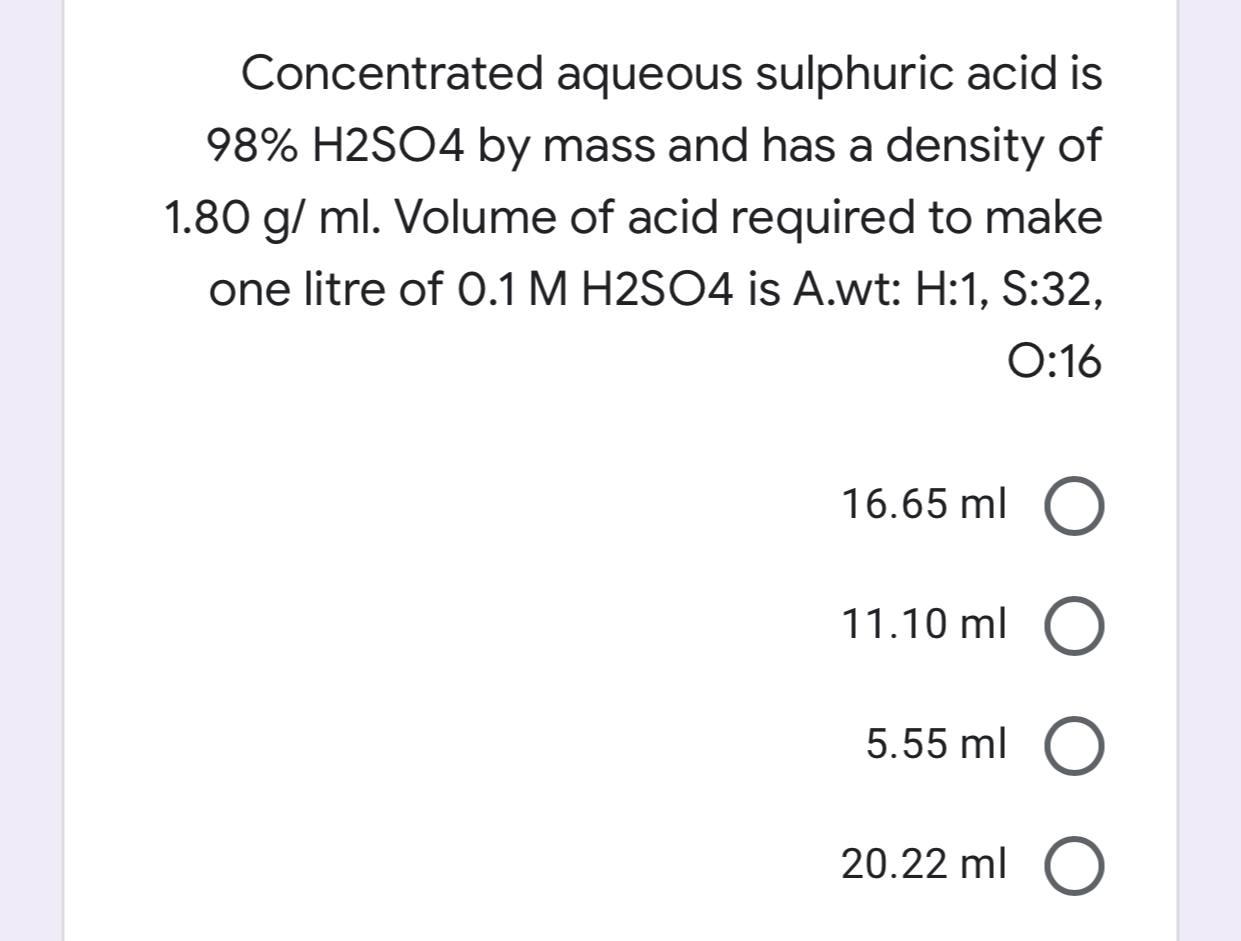
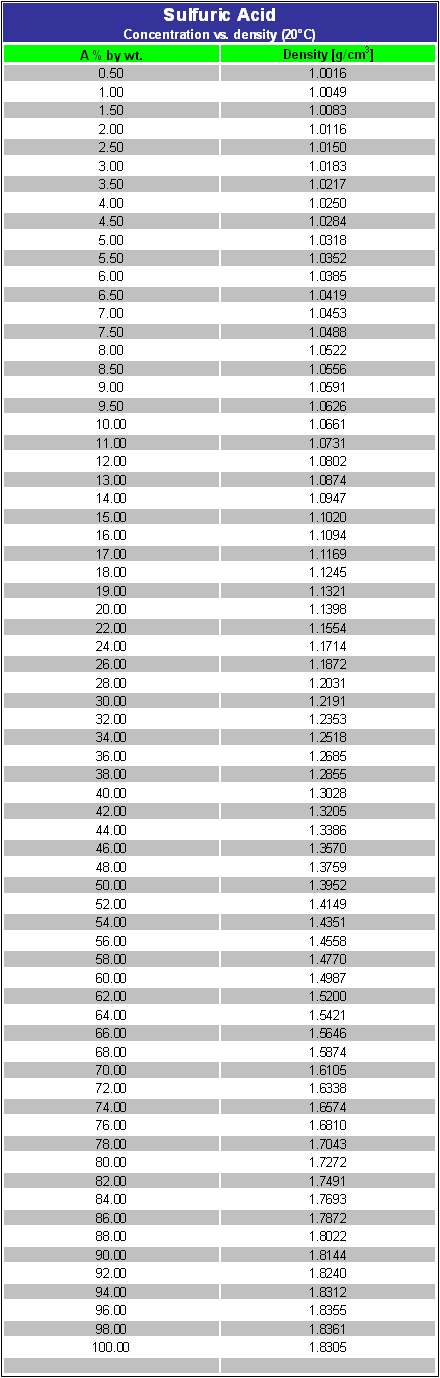
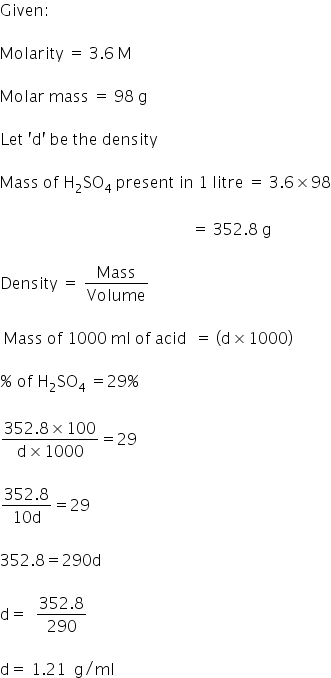What is the volume of concentrated H2SO4 of specific gravity 1.84 and containing 98% H2SO4 by weights that would contain 40 gm of pure H2SO4? - Quora
Chem201, Winter 2006 Name Answer key______________ Midterm N1 01/26/06 SID___________________________ 1. A solution is prepared
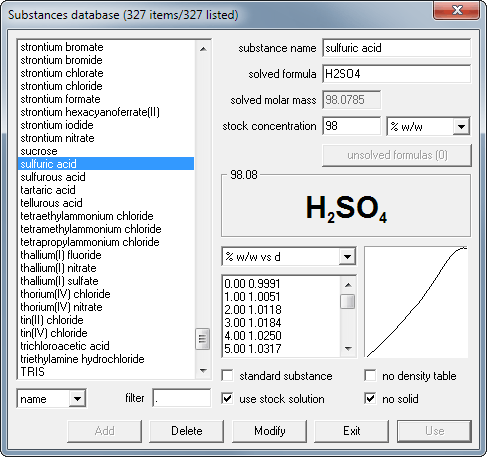
Concentration conversions using CASC - converting molarity to percent concentration, checking density

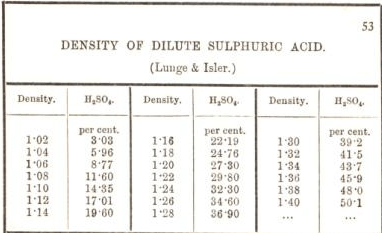
a bottle of concentrated sulphuric acid (density of 1.80 g cm-3) is labelled as 86% as weight. What is - Brainly.in

Kinetics of selenium and tellurium removal with cuprous ion from copper sulfate-sulfuric acid solution | Semantic Scholar
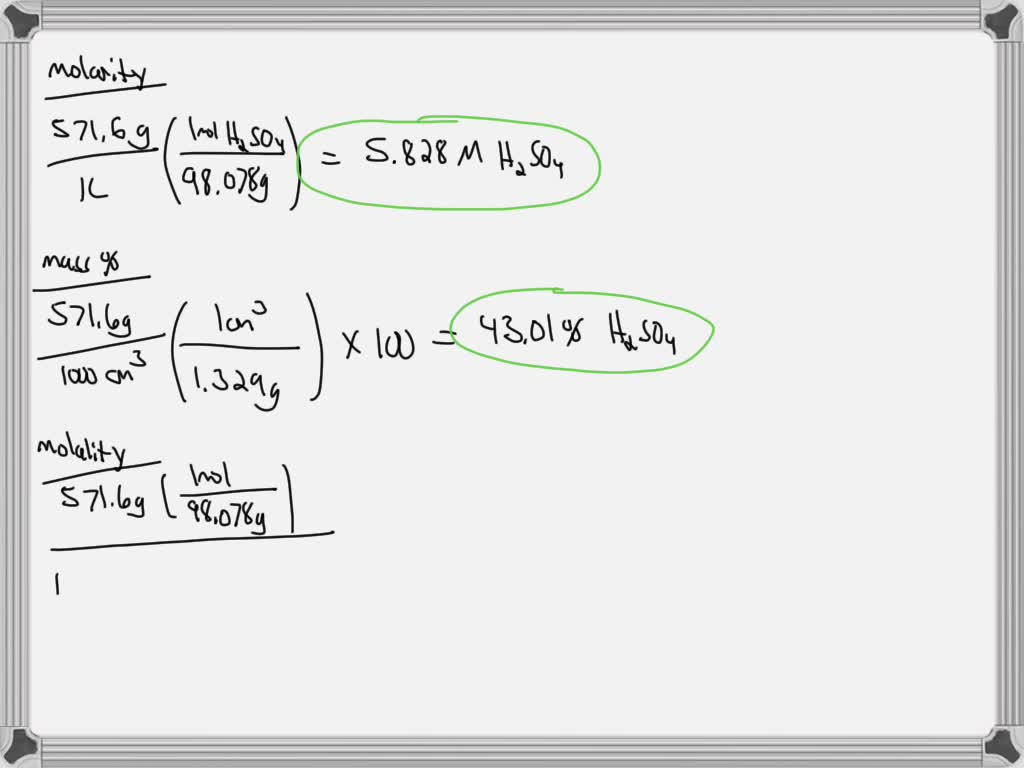
SOLVED: Molarity of sulphuric acid is 0.8 and its density is 1.06 gram per centimetre cube What will be its concentration in terms of molarity and mole fraction? A. 0.785 mole per
The density (in g mL^–1 ) of a 3.60M sulphuric acid solution that is 29% H2SO4 (molar mass = 98g mol^–1 ) by mass will be. - Sarthaks eConnect | Largest Online Education Community

What volume of 95% sulphuric acid (density = 1.85 g mL^(-1)) and what mass of water must be taken to prepare 100 mL of 15% solution of sulphuric acid ( density = 1.1 g mL^(-1)) ?