Dehydroacetic acid and its sodium salt: Human health tier II assessment Chemicals in this assessment Preface
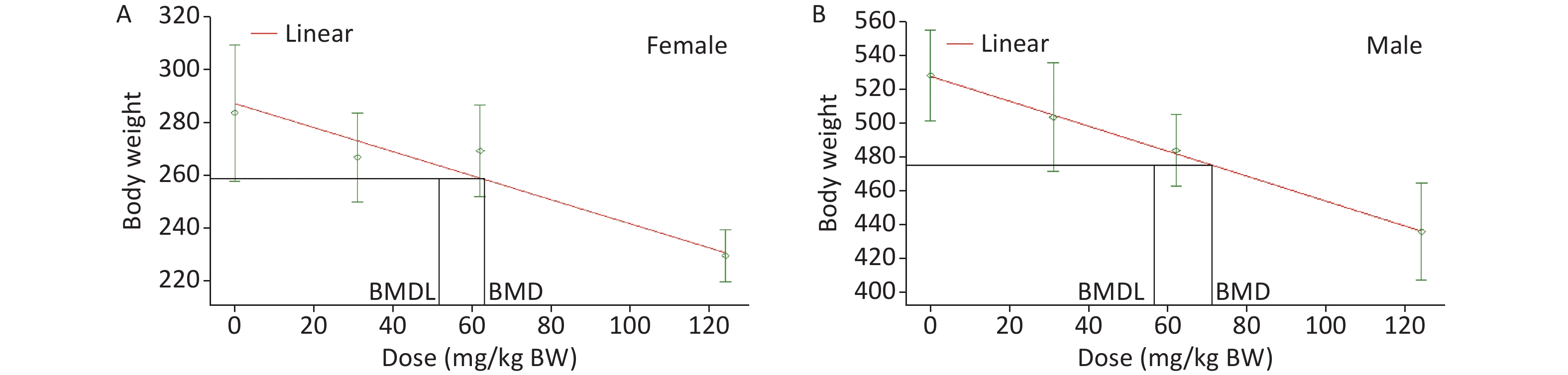
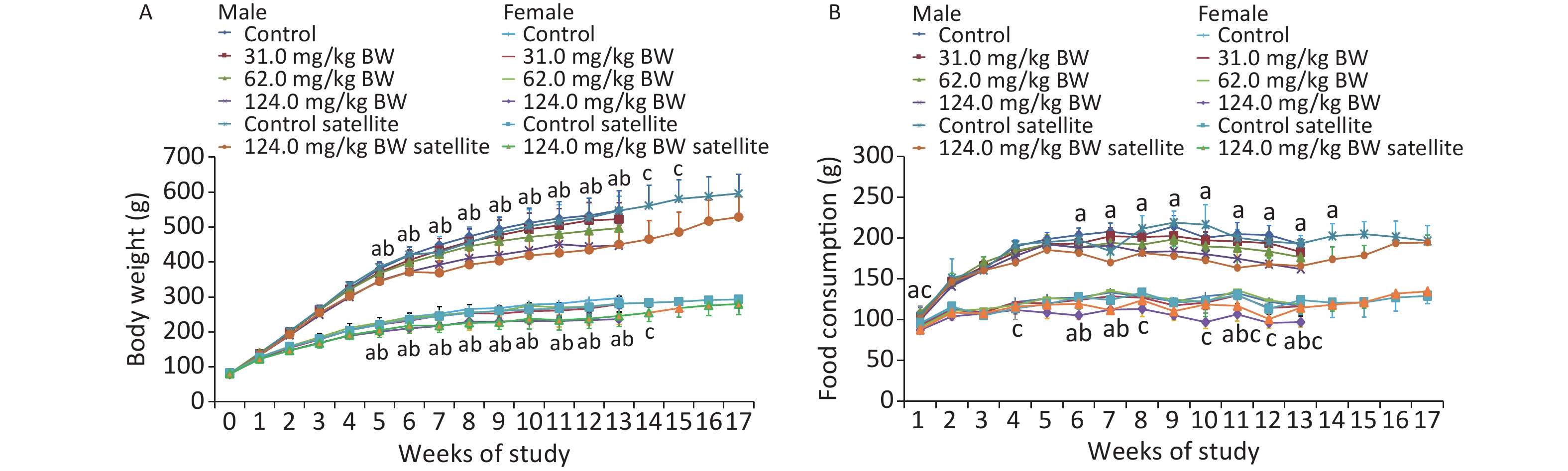
Original Article Subchronic Oral Toxicity Evaluation of Sodium Dehydroacetate: A 90-day Repeated Dose Study in Rats*

Integrated approaches to testing and assessment as a tool for the hazard assessment and risk characterization of cosmetic preservatives - Canavez - 2021 - Journal of Applied Toxicology - Wiley Online Library

A repeated dose 28-day oral toxicity study of sodium dehydroacetate (DHA-S) in Wistar rats - ScienceDirect

A repeated dose 28-day oral toxicity study of sodium dehydroacetate (DHA-S) in Wistar rats - ScienceDirect
Dehydroacetic acid and its sodium salt: Human health tier II assessment Chemicals in this assessment Preface
Original Article Subchronic Oral Toxicity Evaluation of Sodium Dehydroacetate: A 90-day Repeated Dose Study in Rats*