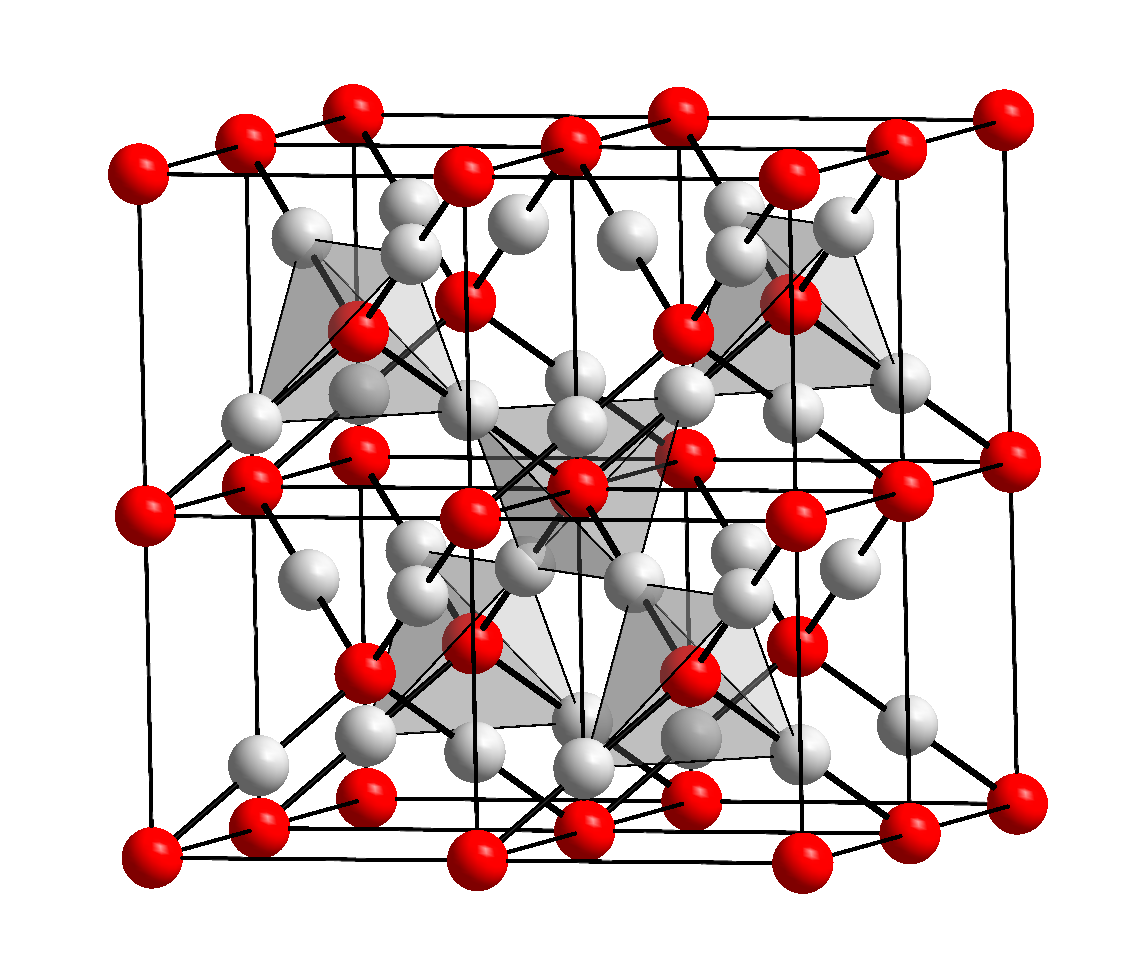
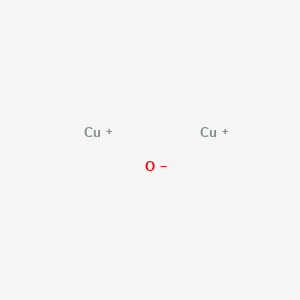
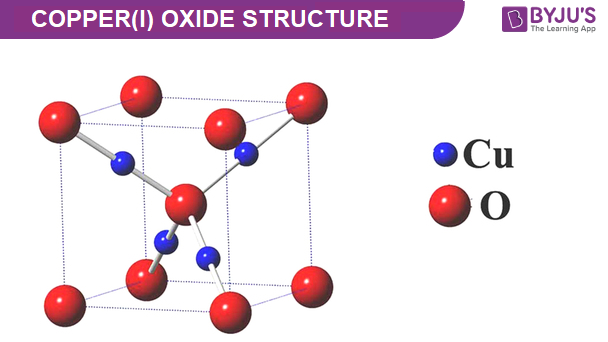
Design, Growth, and Characterization of Crystalline Copper Oxide p-Type Transparent Semiconductive Thin Films with Figures of Merit Suitable for Their Incorporation into Translucent Devices | Crystal Growth & Design
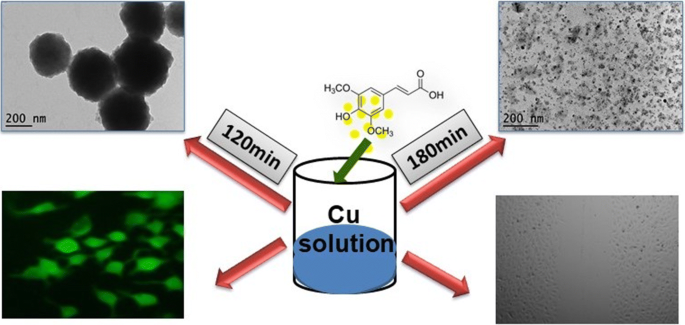
Green synthesis of copper oxide nanoparticles using sinapic acid: an underpinning step towards antiangiogenic therapy for breast cancer | SpringerLink

Charge Delocalization, Oxidation States, and Silver Mobility in the Mixed Silver–Copper Oxide AgCuO2 | Inorganic Chemistry

Kupfer(I)oxid Rot, Cu2O, min. 97,0%, cuprous oxide, 1317-39-1, Dikupferoxid (25,0kg) : Amazon.de: Gewerbe, Industrie & Wissenschaft

Water tolerant base free Copper (I) catalyst for the selective aerobic oxidation of primary alcohols - ScienceDirect

Wholesale CAS:1317-38-0 | High Quality Cupric Oxide Copper Oxide Manufacturer and Supplier | Hongyuan New Materials