
Perphenazine–Macrocycle Conjugates Rapidly Sequester the Aβ42 Monomer and Prevent Formation of Toxic Oligomers and Amyloid | ACS Chemical Neuroscience

Rational design of a conformation-specific antibody for the quantification of Aβ oligomers. - Abstract - Europe PMC

An anticancer drug suppresses the primary nucleation reaction that initiates the production of the toxic Aβ42 aggregates linked with Alzheimer's disease | Science Advances

PDF) Small molecule-mediated co-assembly of amyloid-β oligomers reduces neurotoxicity through promoting non-fibrillar aggregation

Small-molecule sequestration of amyloid-β as a drug discovery strategy for Alzheimer's disease | Science Advances
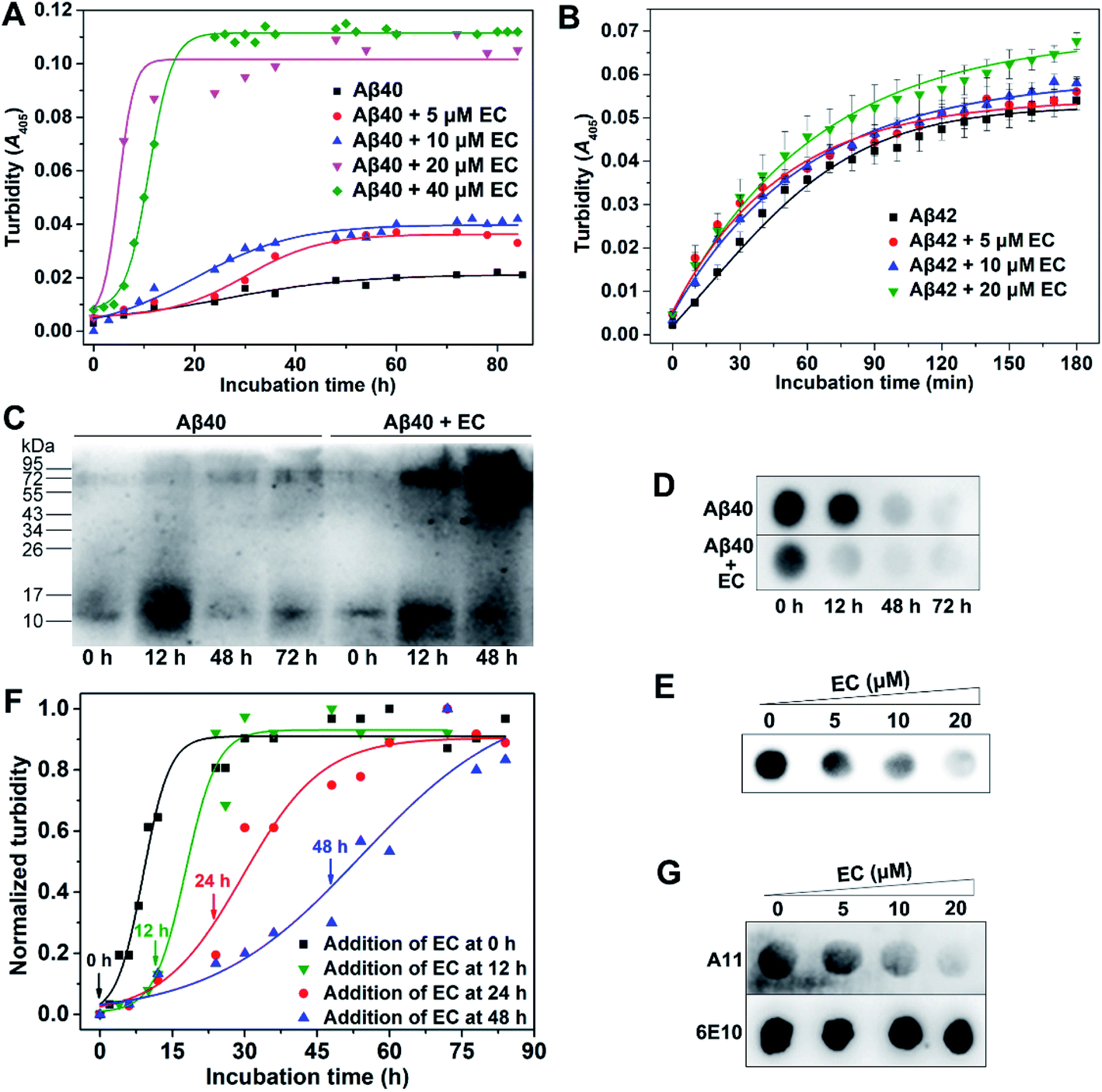
Small molecule-mediated co-assembly of amyloid-β oligomers reduces neurotoxicity through promoting non-fibrillar aggregation - Chemical Science (RSC Publishing) DOI:10.1039/D0SC00392A

Moxifloxacin Disrupts and Attenuates Aβ42 Fibril and Oligomer Formation: Plausibly Repositioning an Antibiotic as Therapeutic against Alzheimer's Disease | ACS Chemical Neuroscience

An anticancer drug suppresses the primary nucleation reaction that initiates the production of the toxic Aβ42 aggregates linked with Alzheimer's disease | Science Advances
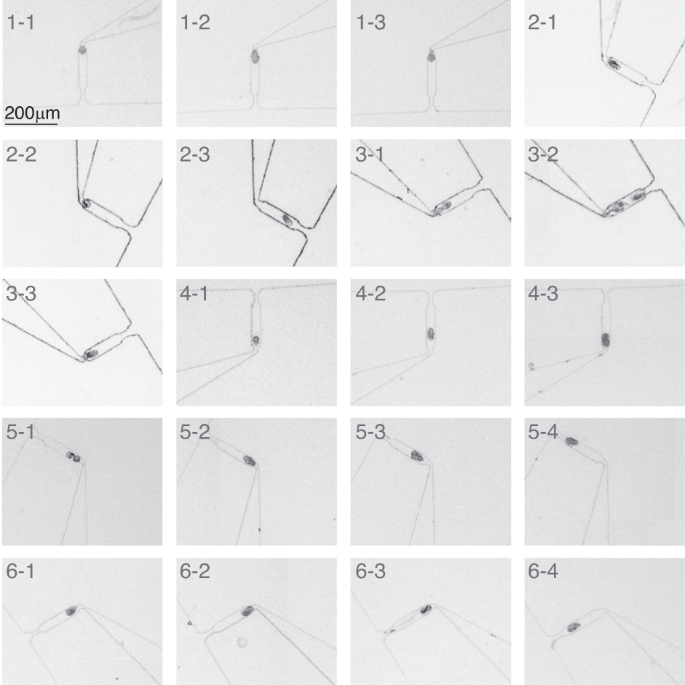
A spiral microfluidic device for rapid sorting, trapping, and long-term live imaging of Caenorhabditis elegans embryos | Microsystems & Nanoengineering

Small-molecule sequestration of amyloid-β as a drug discovery strategy for Alzheimer's disease | Science Advances
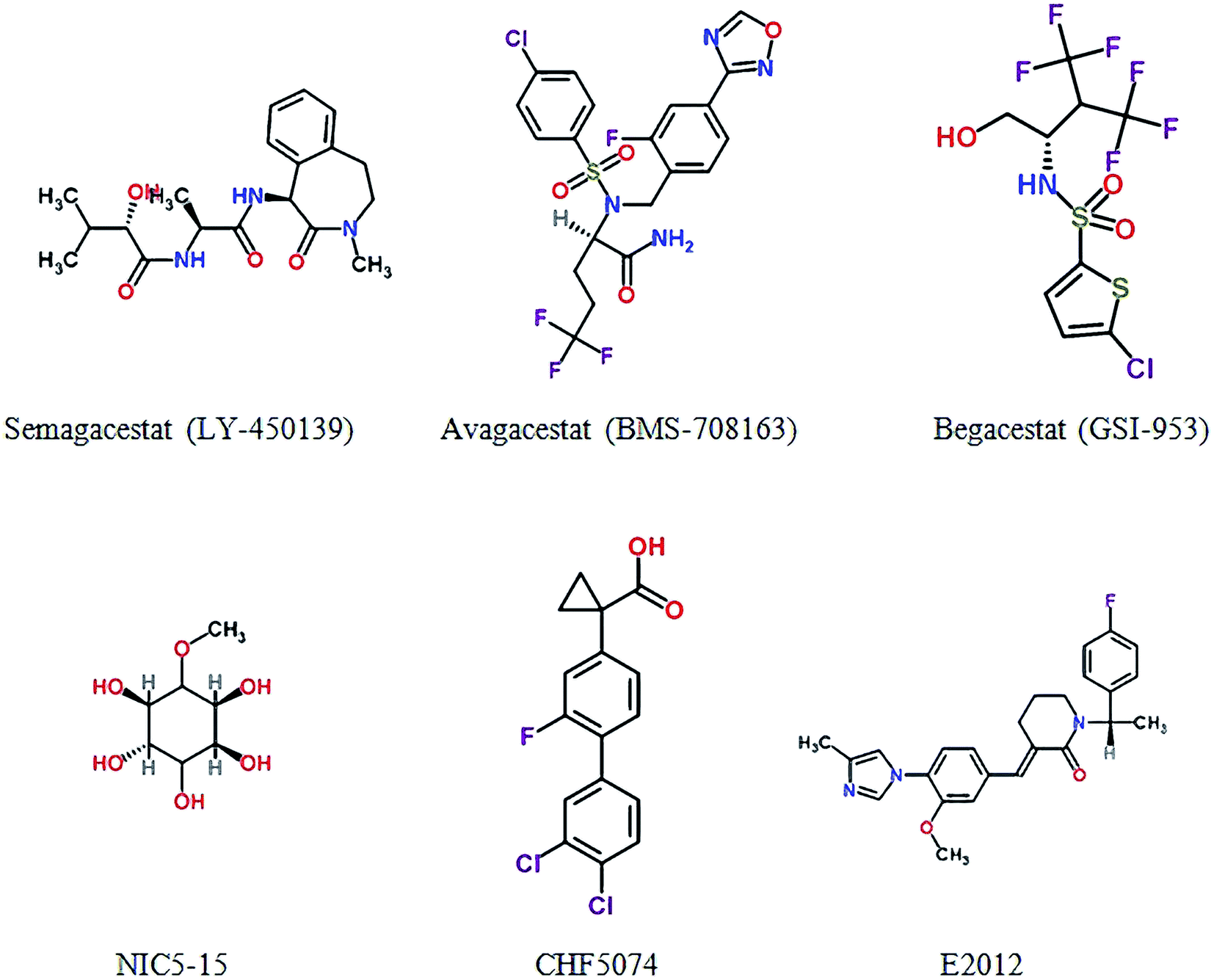
Two decades of new drug discovery and development for Alzheimer's disease - RSC Advances (RSC Publishing) DOI:10.1039/C6RA26737H
PDF) A rationally designed bicyclic peptide remodels Aβ42 aggregation in vitro and reduces its toxicity in a worm model of Alzheimer's disease
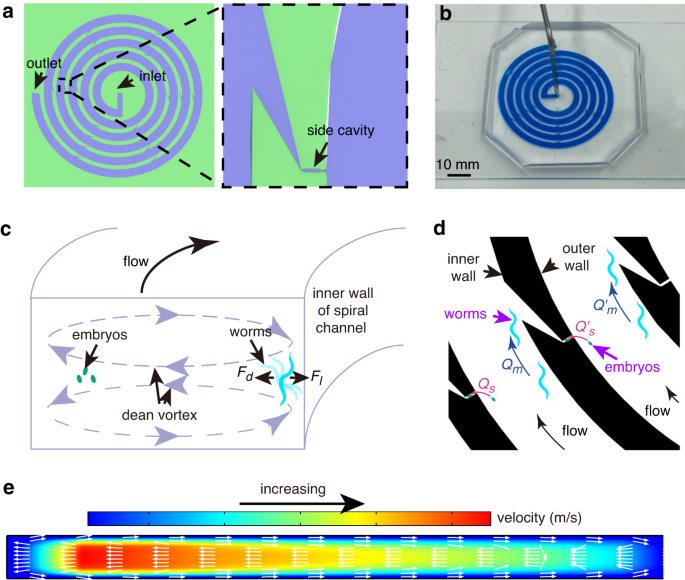
A spiral microfluidic device for rapid sorting, trapping, and long-term live imaging of Caenorhabditis elegans embryos | Microsystems & Nanoengineering
PDF) Exogenous misfolded protein oligomers can cross the intestinal barrier and cause a disease phenotype in C. elegans

Perphenazine–Macrocycle Conjugates Rapidly Sequester the Aβ42 Monomer and Prevent Formation of Toxic Oligomers and Amyloid | ACS Chemical Neuroscience

An anticancer drug suppresses the primary nucleation reaction that initiates the production of the toxic Aβ42 aggregates linked with Alzheimer's disease | Science Advances

Selective targeting of primary and secondary nucleation pathways in Aβ42 aggregation using a rational antibody scanning method | Science Advances
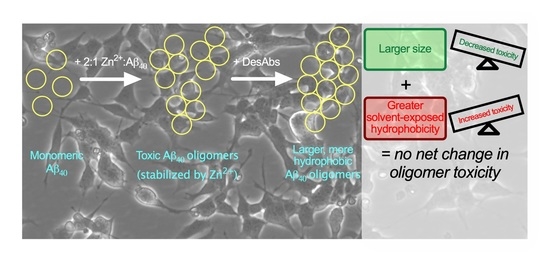
IJMS | Free Full-Text | Rationally Designed Antibodies as Research Tools to Study the Structure–Toxicity Relationship of Amyloid-β Oligomers

Drug discovery: Insights from the invertebrate Caenorhabditis elegans - Giunti - 2021 - Pharmacology Research & Perspectives - Wiley Online Library
PDF) Delivery of Native Proteins into C. Elegans Using a Transduction Protocol Based on Lipid Vesicles

Genetic and Pharmacological Discovery for Alzheimer's Disease Using Caenorhabditis elegans | ACS Chemical Neuroscience

PDF) Systematic development of small molecules to inhibit specific microscopic steps of Aβ42 aggregation in Alzheimer's disease
![Hexahydropyrrolo[2,3-b]indole Compounds as Potential Therapeutics for Alzheimer's Disease | ACS Chemical Neuroscience Hexahydropyrrolo[2,3-b]indole Compounds as Potential Therapeutics for Alzheimer's Disease | ACS Chemical Neuroscience](https://pubs.acs.org/cms/10.1021/acschemneuro.9b00297/asset/images/medium/cn9b00297_0007.gif)

