physical chemistry - Which make HCO3- to show two pH values at two scenarios? - Chemistry Stack Exchange

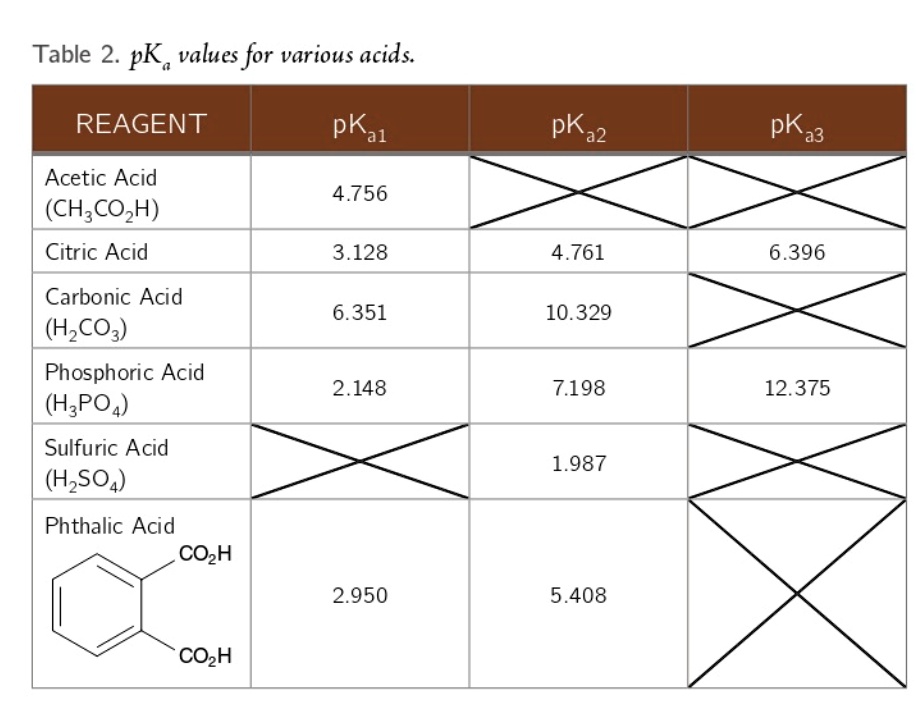
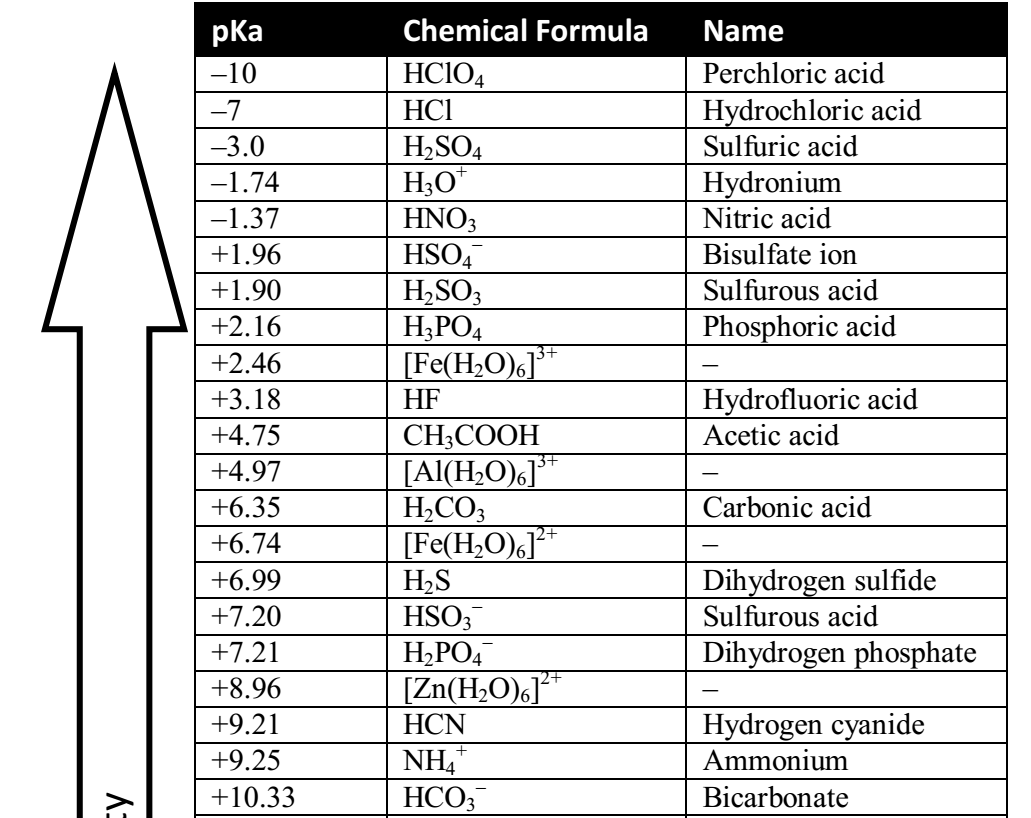
OneClass: Calculate the ratio of bicarbonate to carbonic acid at pH 7.4,using the following pKa value...

Biological buffering of blood There are three major contributors to regulating the pH of blood. Bicarbonate, phosphate and proteins Blood pH Must be Kept. - ppt download
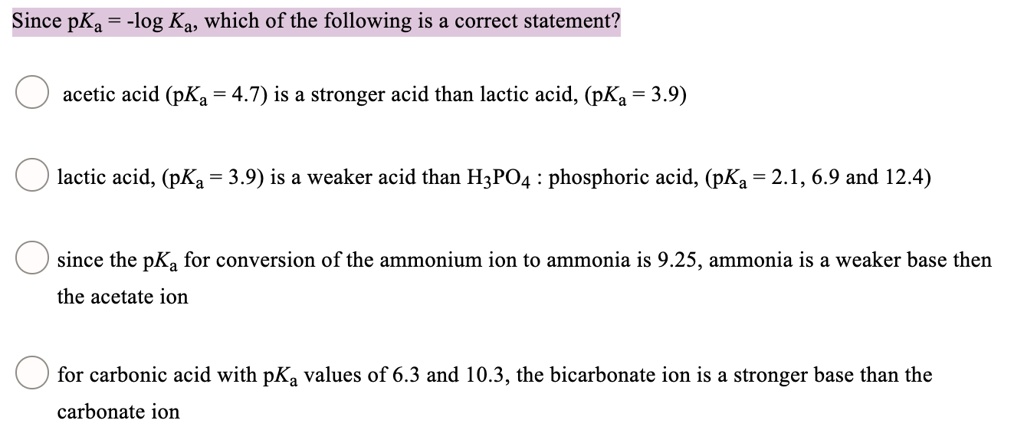
SOLVED: Since pKa -log Ka; which of the following is a correct statement? acetic acid (pKa = 4.7) is a stronger acid than lactic acid, (pKa 3.9) lactic acid, (pKa = 3.9)
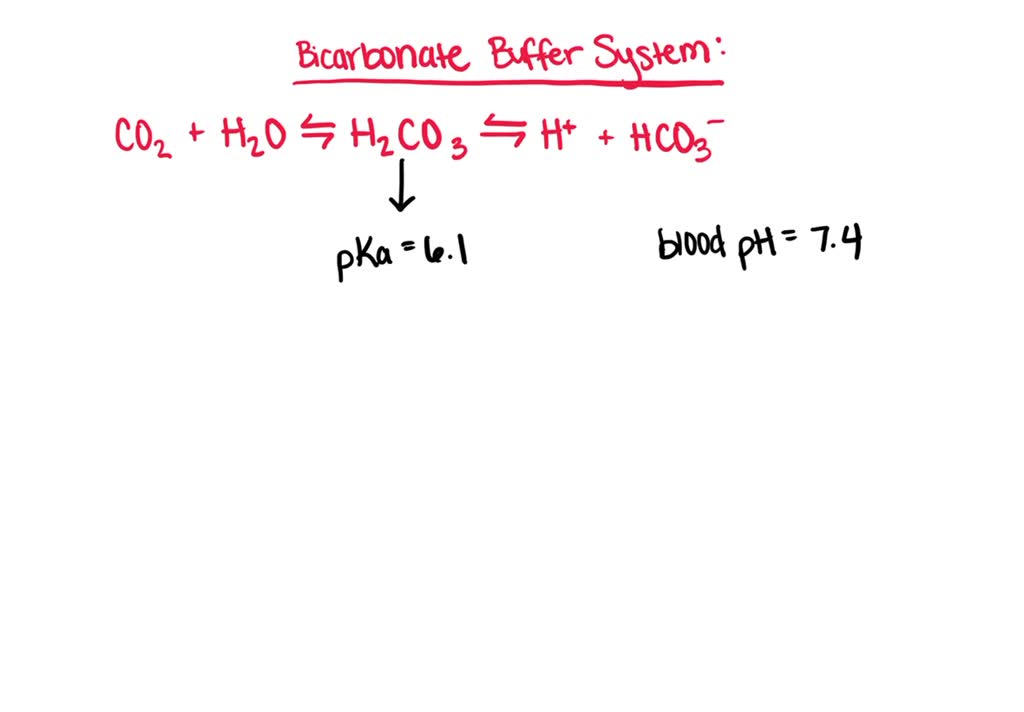
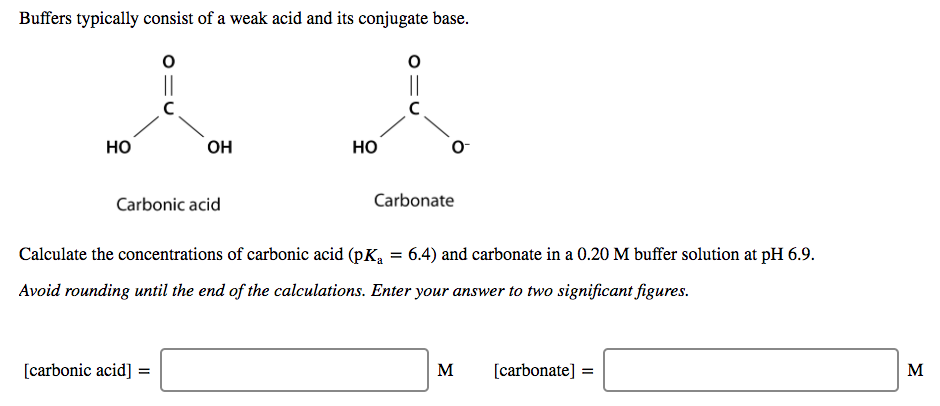
SOLVED: Carbonic acid has a pKa of 6.1 at physiological temperature. Is the carbonic acid/bicarbonate buffer system that maintains the pH of the blood at 7.4 better at neutralizing excess acid or

The comparison of pKa determination between carbonic acid and formic acid and its application to prediction of the hydration numbers - ScienceDirect
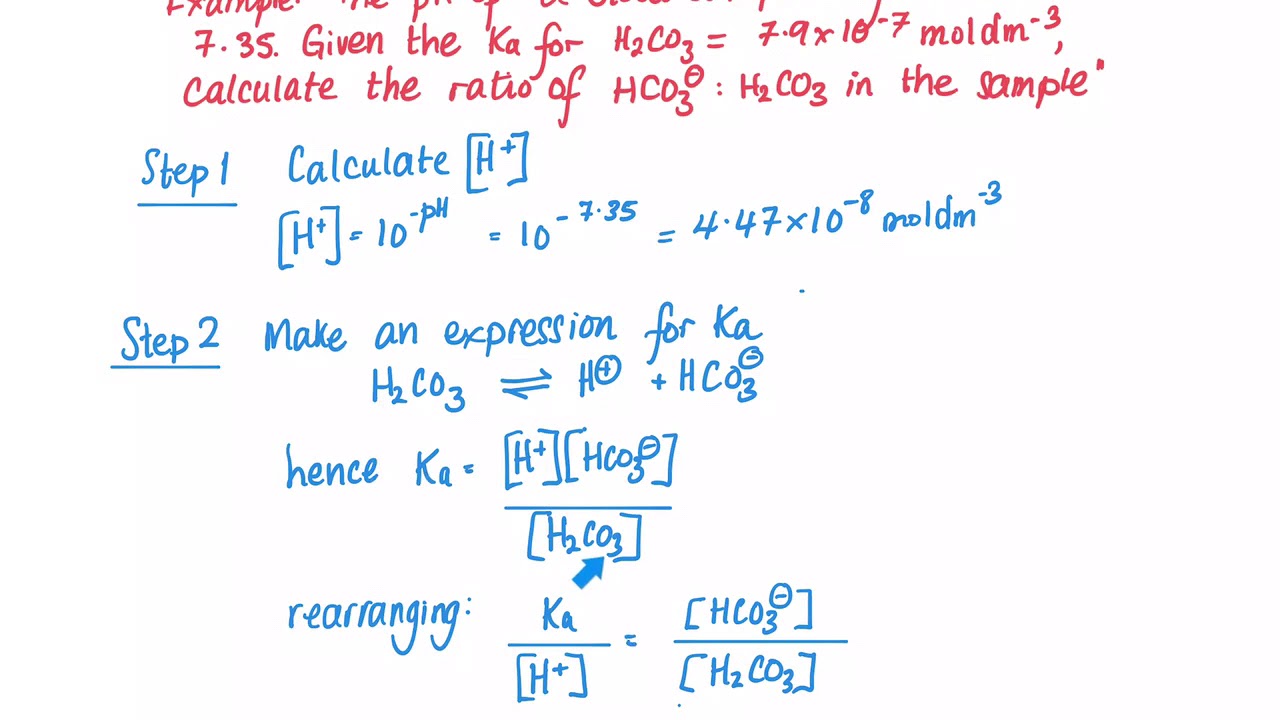
Why, when CO2 is accumulated in our body, does it turn to H2CO3, and then dissociate to HCO3- and H+ (? Why does this acid dissociate and increase H+, for no purpose,
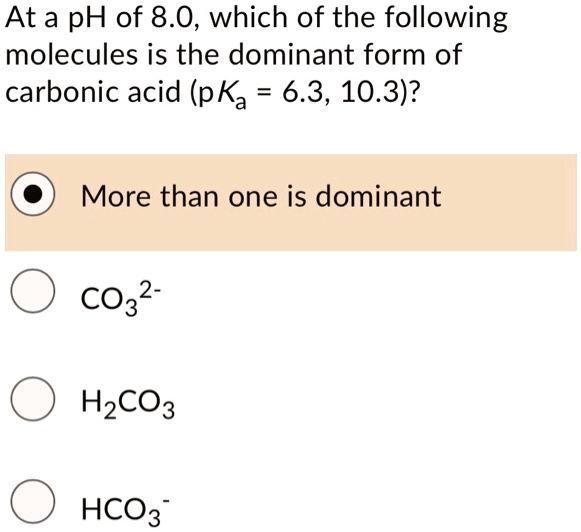
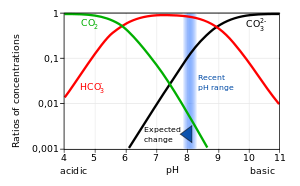
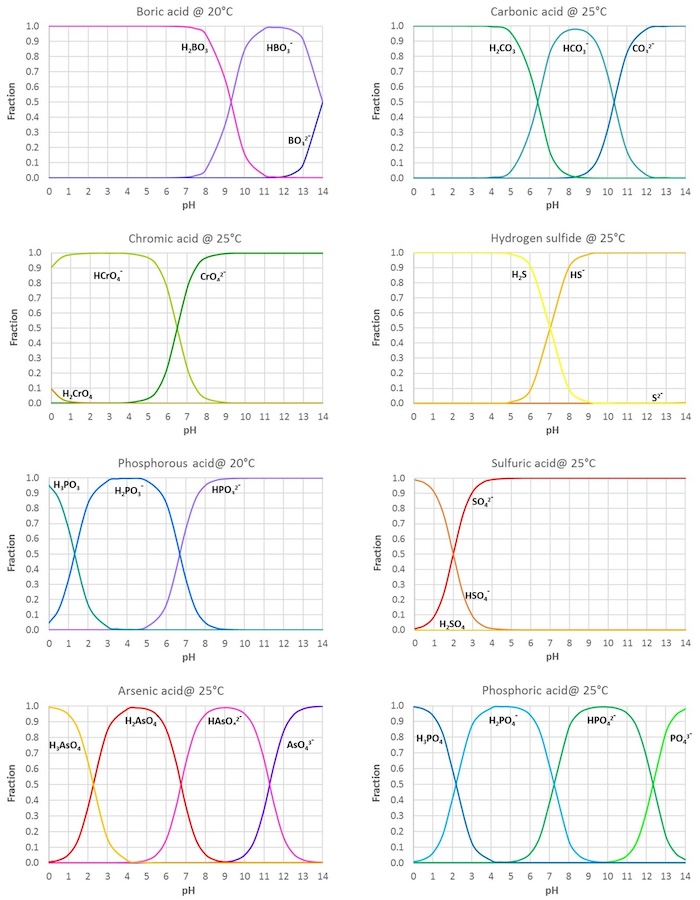
SOLVED: At a pH of 8.0, which of the following molecules is the dominant form of carbonic acid (pKa = 6.3, 10.3)? More than one is dominant CO3 2 - H2CO3 HCO3