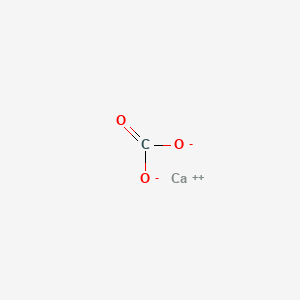
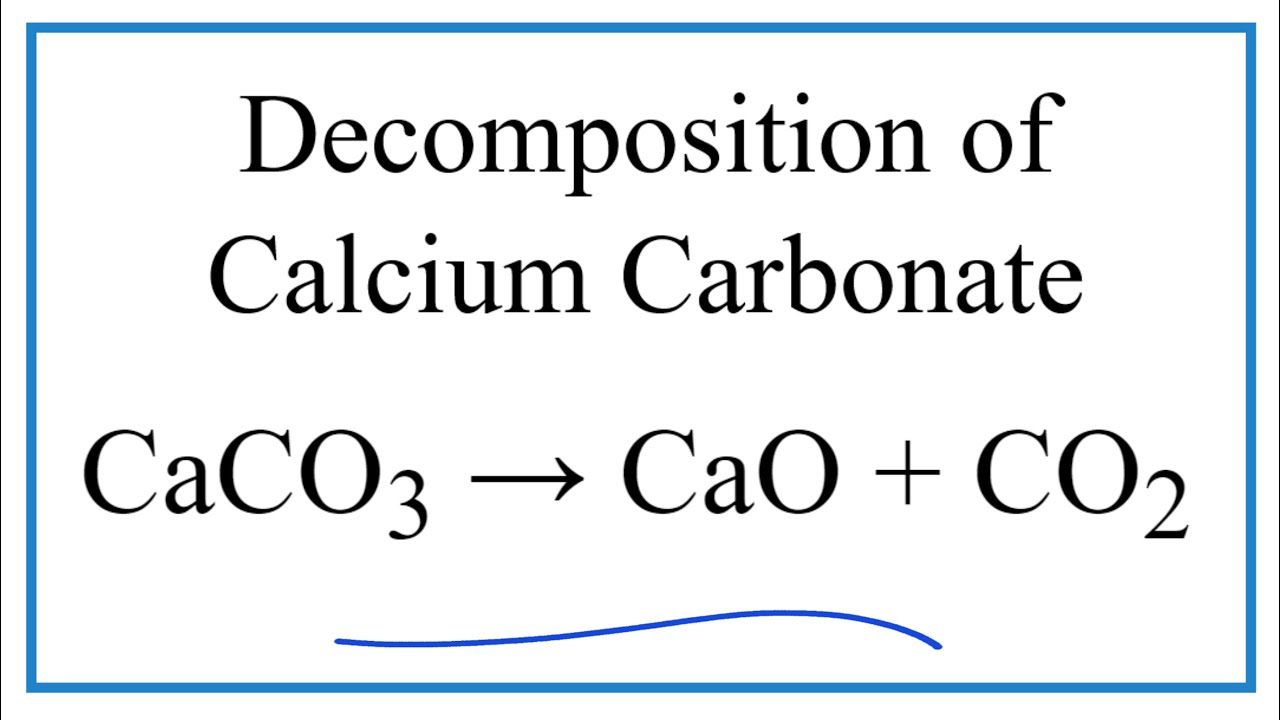
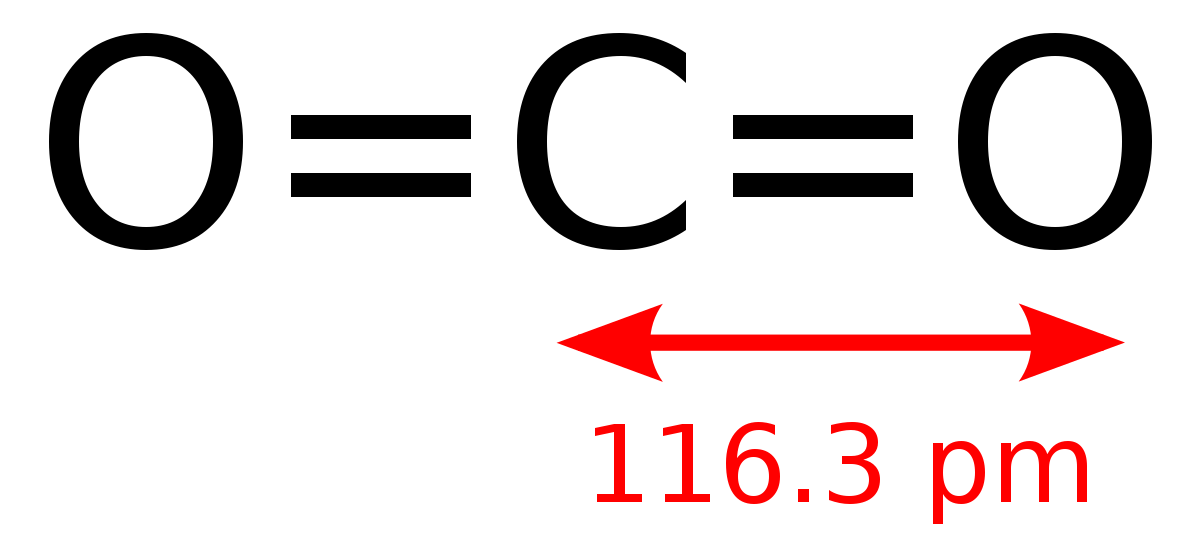
Question Video: Calculating the the Enthalpy Change for the Thermal Decomposition of Lithium Carbonate Using Enthalpies of Formation | Nagwa
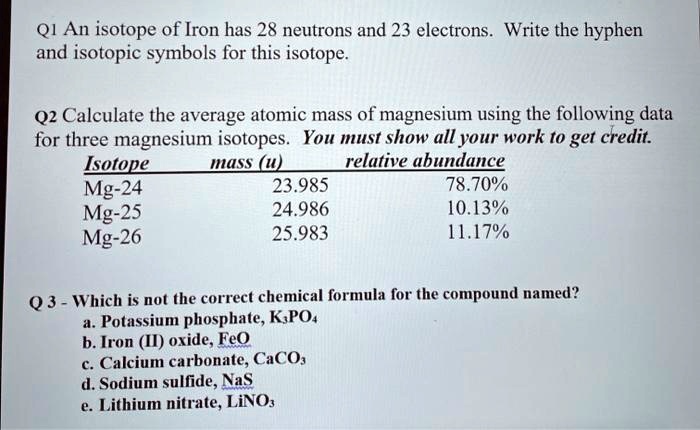
SOLVED: Q1 An isotope of Iron has 28 neutrons and 23 electrons. Write the hyphen and isotopic symbols for this isotope Q2 Calculate the average atomic mass of magnesium using the following

Zinc oxide reacts whith carbon, on heating, to from zinc metal and carbon monoxide. Write a balanced - YouTube
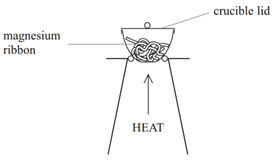
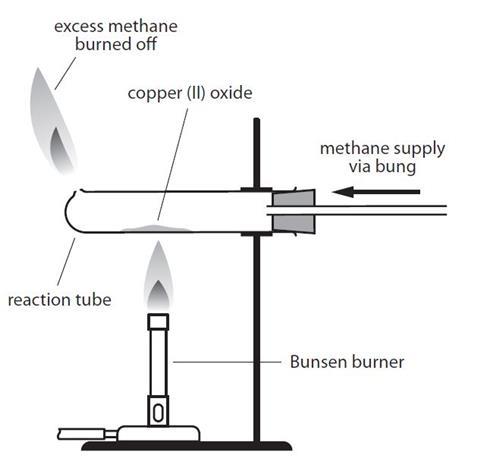
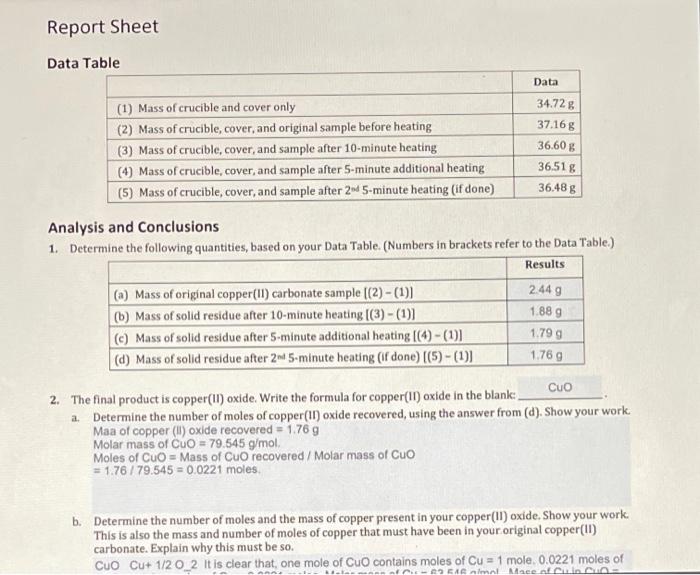
1:36 practical: know how to determine the formula of a metal oxide by combustion (e.g. magnesium oxide) or by reduction (e.g. copper(II) oxide) - TutorMyself Chemistry

Sodium Oxide (Na2O) - Structure, Physical Properties, Chemical Properties and Uses with FAQs of Sodium Oxide