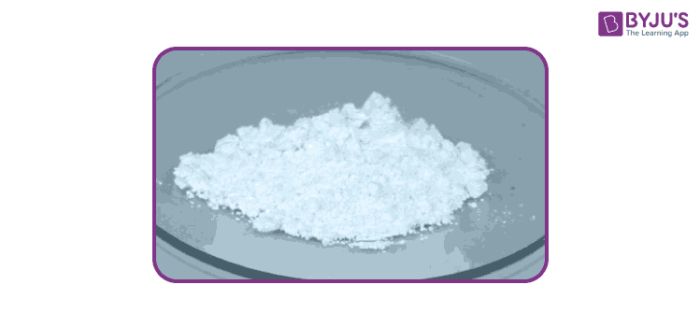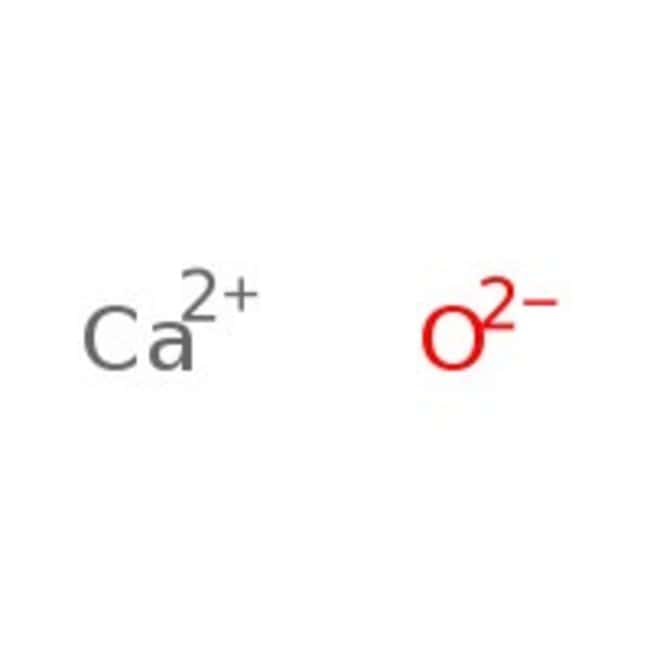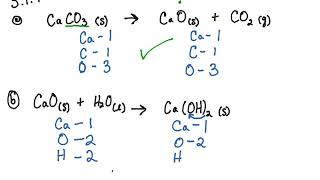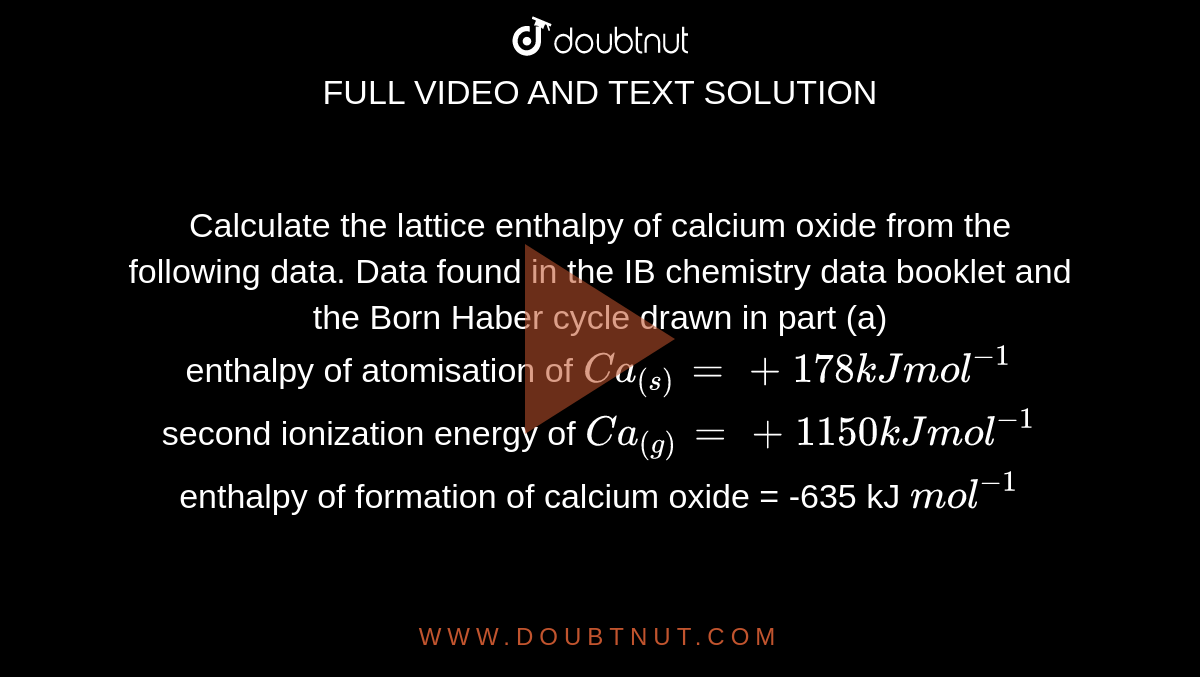
Calculate the lattice enthalpy of calcium oxide from the following data. Data found in the IB chemistry data booklet and the Born Haber cycle drawn in part (a) enthalpy of atomisation of
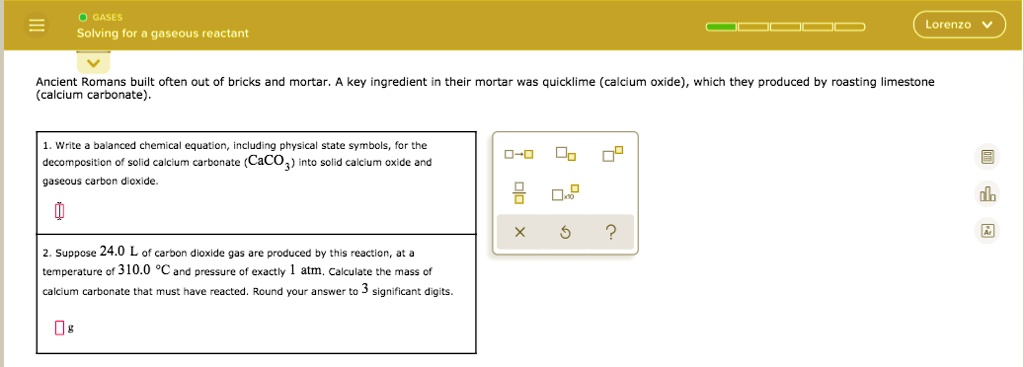
SOLVED: GASES Solving Ior gjascous reuctant Lorenzo Ancient Romans built often out of bricks and mortar, key ingredient their mortar was quicklime (calcium oxide) , "hich they produced bY roasting limestone (calcium

Question Video: Calculating the Mass of Calcium Carbonate Required to Produce a Given Mass of Calcium Oxide | Nagwa

SOLVED: Caldum oxide reacts with water in combination reaction produce calcium hydroxide; CaO (s) H2O (I) Ca(OH)2 (s) 3.50-8 sample of CaO is reacted with 3.38 gof HzO How Many grams of
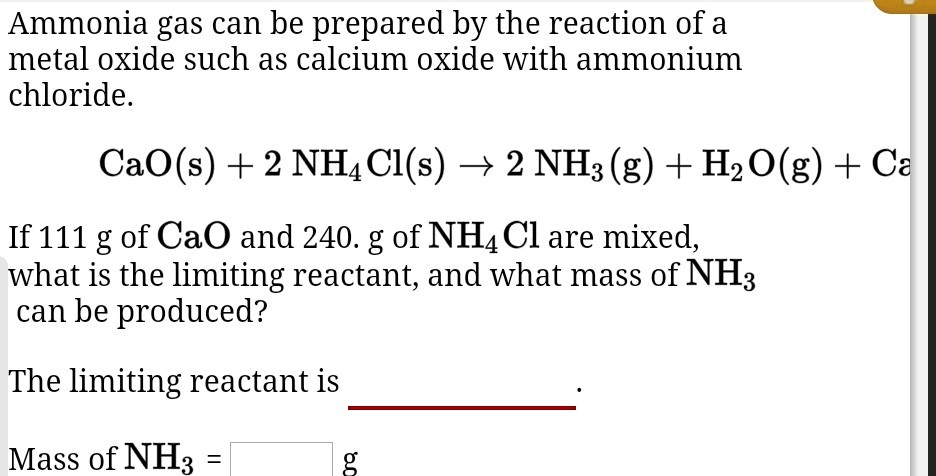
SOLVED: Ammonia gas can be prepared by the reaction of a metal oxide such as calcium oxide with ammonium chloride: CaO(s) + 2 NH4 Cl(s) 72 NH (g) + HzO(g) + Ca
![SOLVED: CaCO3(heat) = CaO + CO2 Determine the ∆G for the reaction of CaCO3(s) forming solid calcium oxide at 35° C. The concentrations of [CaCO3], [CaO], and [CO2] are 0.15 M, 0.25 SOLVED: CaCO3(heat) = CaO + CO2 Determine the ∆G for the reaction of CaCO3(s) forming solid calcium oxide at 35° C. The concentrations of [CaCO3], [CaO], and [CO2] are 0.15 M, 0.25](https://cdn.numerade.com/ask_previews/c929f6cd-30ac-4b5d-81c0-5164da820942_large.jpg)