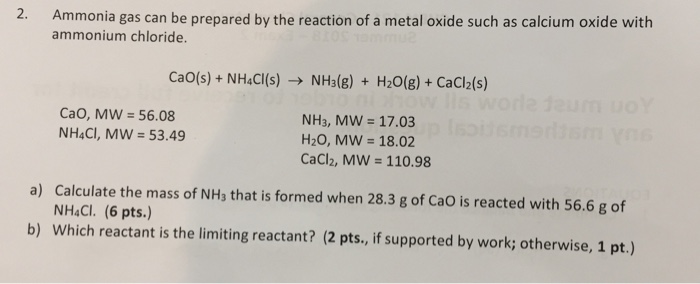Preparation of Ammonia Gas in Laboratory with the Help of Ammonium Chloride and Calcium Oxide Stock Vector - Illustration of white, formula: 220304379
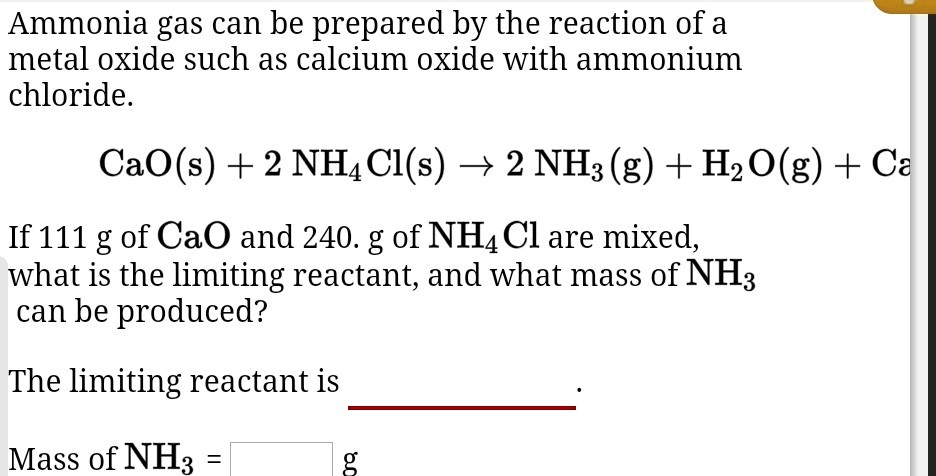
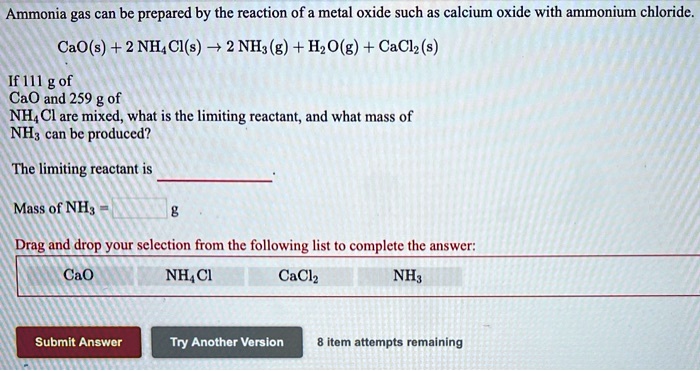
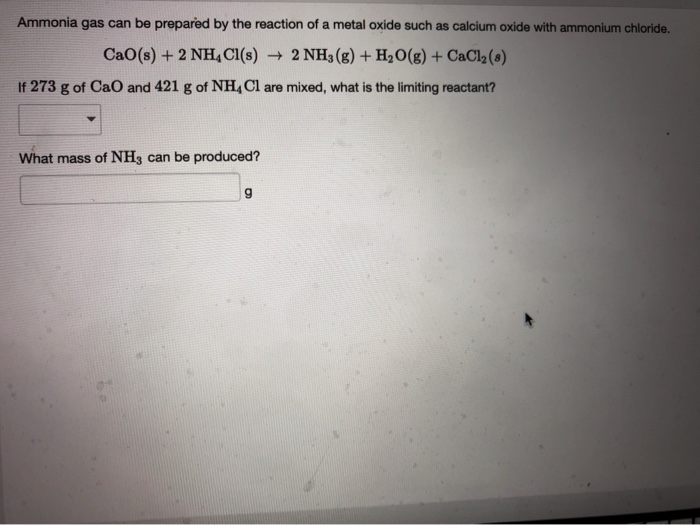
SOLVED: Ammonia gas can be prepared by the reaction of a metal oxide such as calcium oxide with ammonium chloride: CaO(s) + 2 NH4 Cl(s) 72 NH (g) + HzO(g) + Ca
![Why calcium ions do not form precipitate with aqueous ammonia [online video] – O Level Secondary Chemistry Tuition Why calcium ions do not form precipitate with aqueous ammonia [online video] – O Level Secondary Chemistry Tuition](https://i.ytimg.com/vi/rXYJhFZTlTo/maxresdefault.jpg)
Why calcium ions do not form precipitate with aqueous ammonia [online video] – O Level Secondary Chemistry Tuition

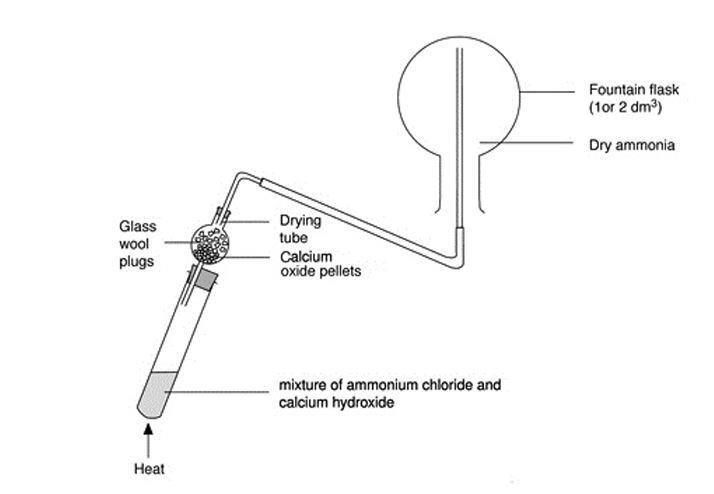
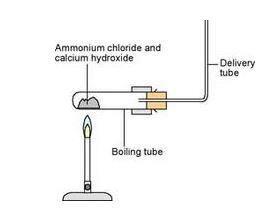
Write the balanced equation for the reaction between a mixture of ammonium chloride and slaked lime.

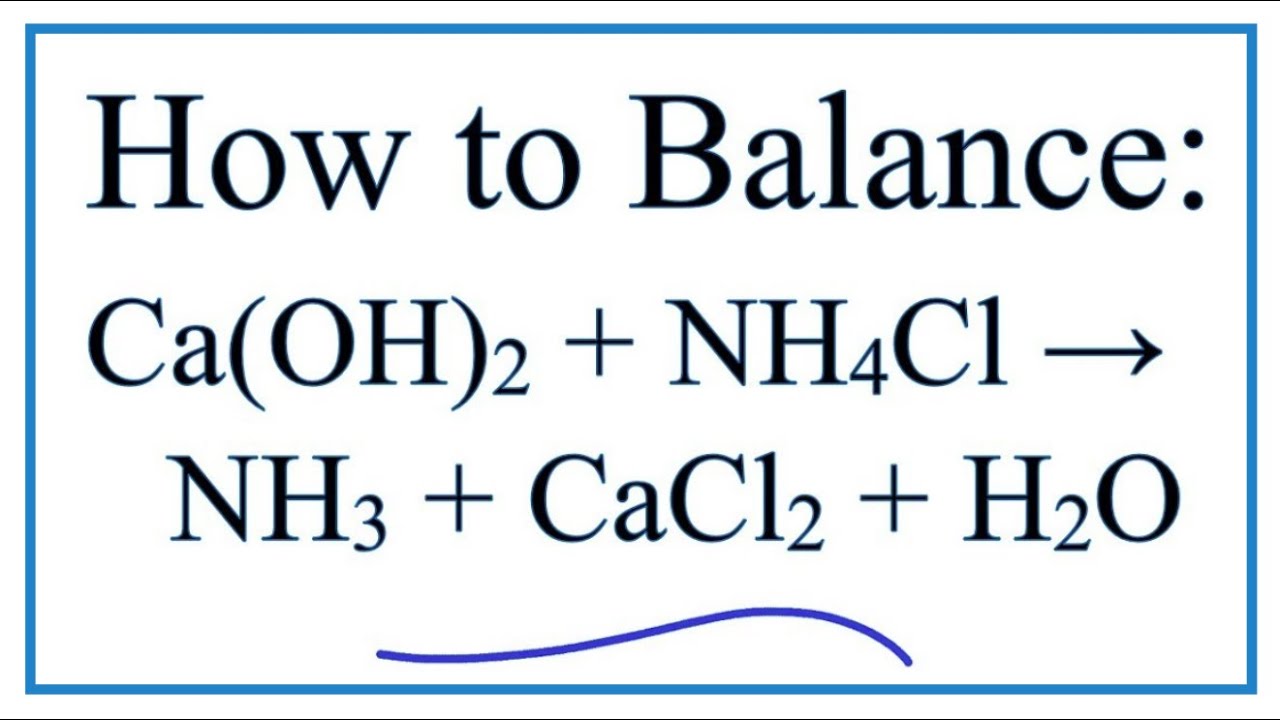
Calcium hydroxide and ammonium chloride react to give ammonia as per equation: Ca(OH)_(2) + 2 NH... - YouTube

List the characteristics of cork. How are they formed? Mention their role.a) Write the formula of (i) Magnesium hydroxide (ii) Hydrogen sulphide (iii) Potassium chloride (iv) Calcium oxide (v) Barium chloride (vi)
![Why calcium ions do not form precipitate with aqueous ammonia [online video] – O Level Secondary Chemistry Tuition Why calcium ions do not form precipitate with aqueous ammonia [online video] – O Level Secondary Chemistry Tuition](https://icandochemistry942105908.files.wordpress.com/2021/10/qa-aq-ammonia.jpg?w=986)
Why calcium ions do not form precipitate with aqueous ammonia [online video] – O Level Secondary Chemistry Tuition

Ammonia, calcium chloride and water are obtained by heating a mixture of ammonium chloride and calcium hydroxide. Write a balanced equation of the reaction.

Toward the Mechanistic Understanding of the Additives' Role on Ammonium Nitrate Decomposition: Calcium Carbonate and Calcium Sulfate as Case Studies | ACS Omega

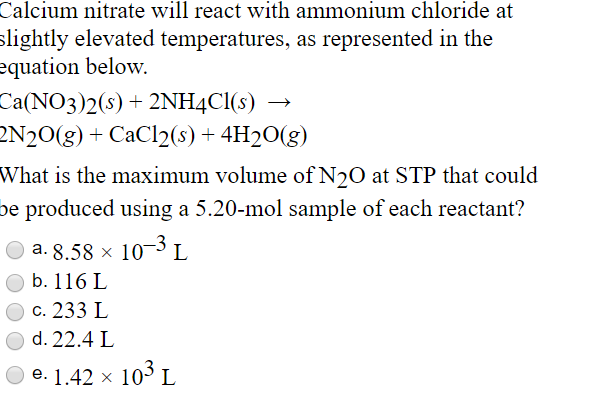
How to Balance the Reaction Between Ammonium Nitrate and Calcium Hydroxide NH4NO3 and Ca(OH)2 - YouTube