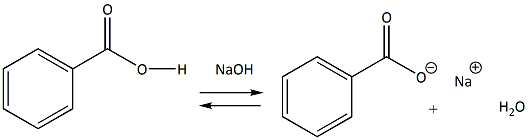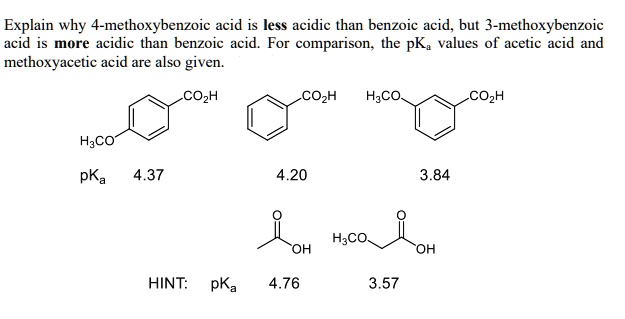
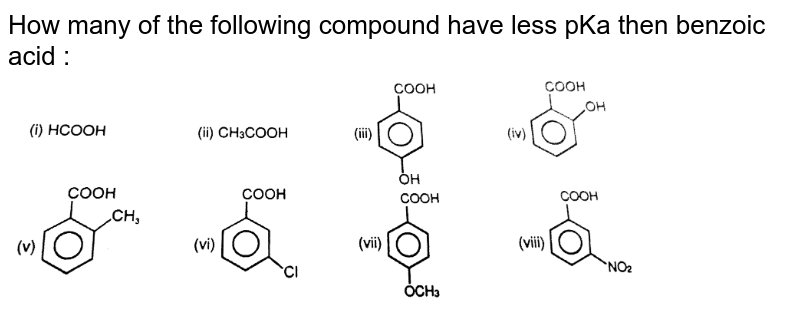
SOLVED: Explain why 4-methoxybenzoic acid is less acidic than benzoic acid, but 3-methoxybenzoic acid is more acidic than benzoic acid. For comparison, the pK values of acetic acid and methoxyacetic acid are

1 M benzoic acid (pKa = 4.2) and 1M C6H5 COONa solutions are given separately. What is the volume of benzoic acid required to prepare a 93 mL buffer solution of pH = 4.5 ?

Calculate the pH of a solution that is made when 0.250 L of 0.20 M benzoic acid (pKa=4.20) and 2.30g - YouTube
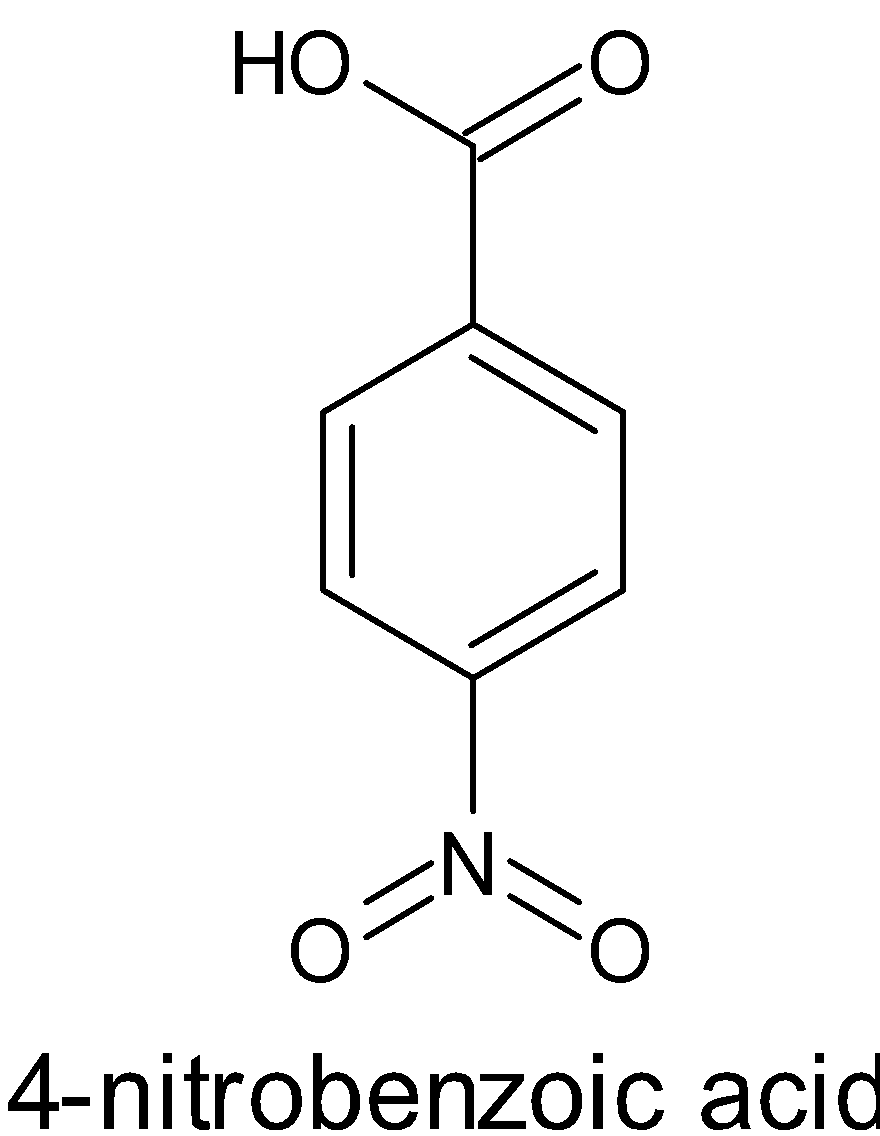
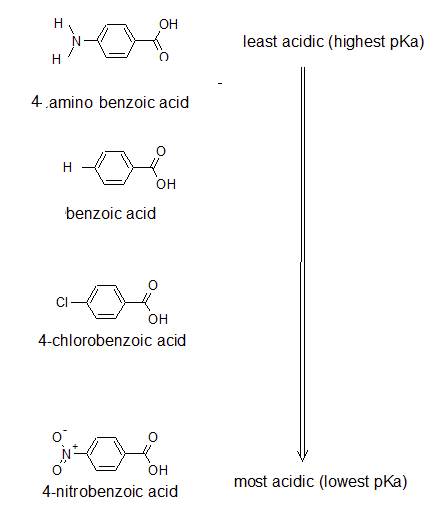
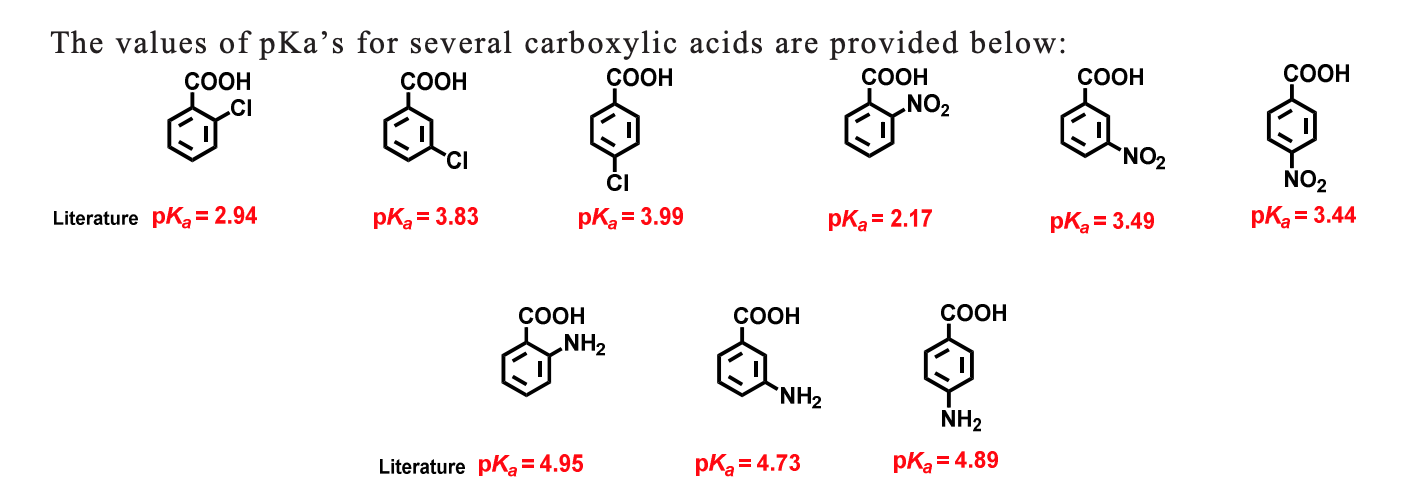
Account for the following:${{p}}{{{K}}_{{a}}}$ value of $4 - $nitrobenzoic acid is lower than that of benzoic acid.

The pKa's of five p-substituted benzoic acids (YC6H4CO2H) are listed below. Rank the corresponding substituted benzenes (YC6H5) in order of their increasing reactivity toward electrophilic aromatic substitution. If benzoic acid has pKa =

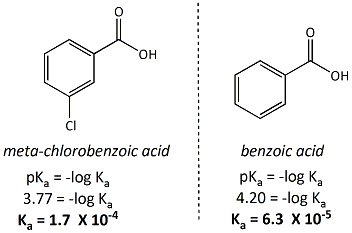
Draw a flowchart that shows how you would seperate benzoic acid from 3,4-dibromophenol. Remember, both compounds are acidic. Look at pKa values to determine which is the most acidic. | Homework.Study.com
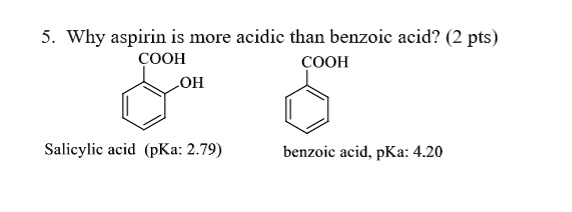
SOLVED: Why aspirin is more acidic than benzoic acid? pts) COOH COOH OH Salicylic acid (pKa: 2.79) benzoic acid. pKa: 4.20

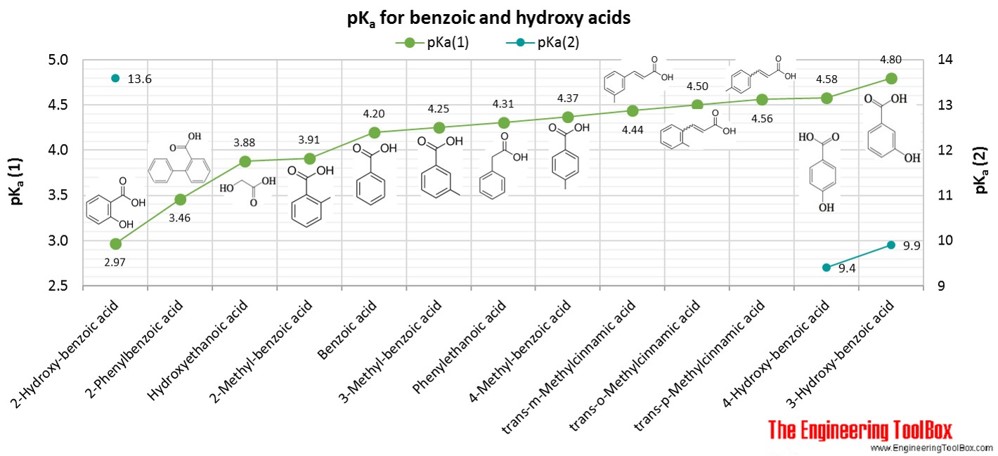
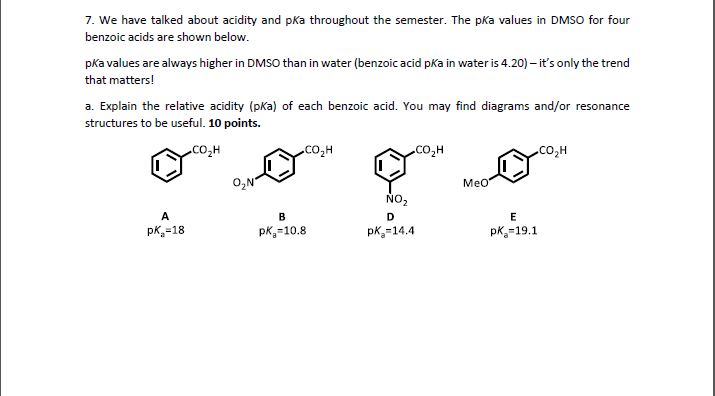
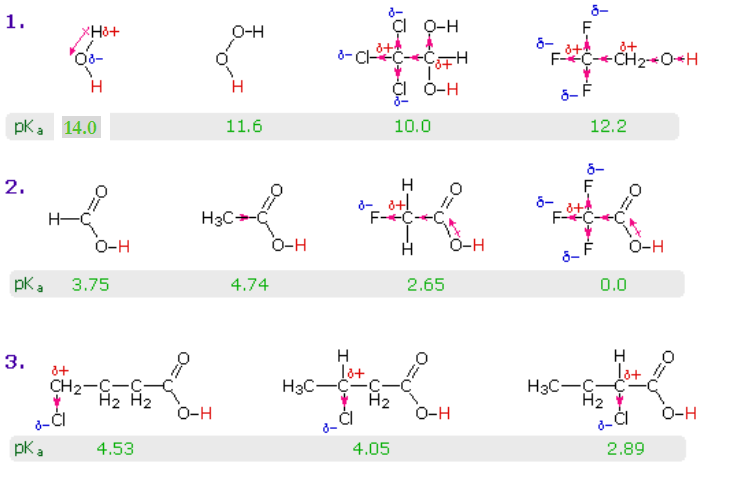
Standard and Absolute pKa Scales of Substituted Benzoic Acids in Room Temperature Ionic Liquids | The Journal of Organic Chemistry

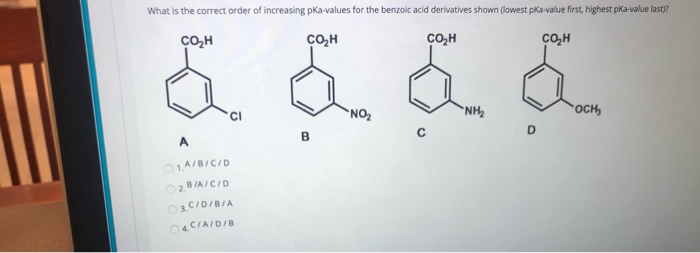
![PDF] Substituent effects on the electronic structure and pKa of benzoic acid | Semantic Scholar PDF] Substituent effects on the electronic structure and pKa of benzoic acid | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/7231c766a16c0fe0c564bc409846e5a74d693a18/4-TableV-1.png)


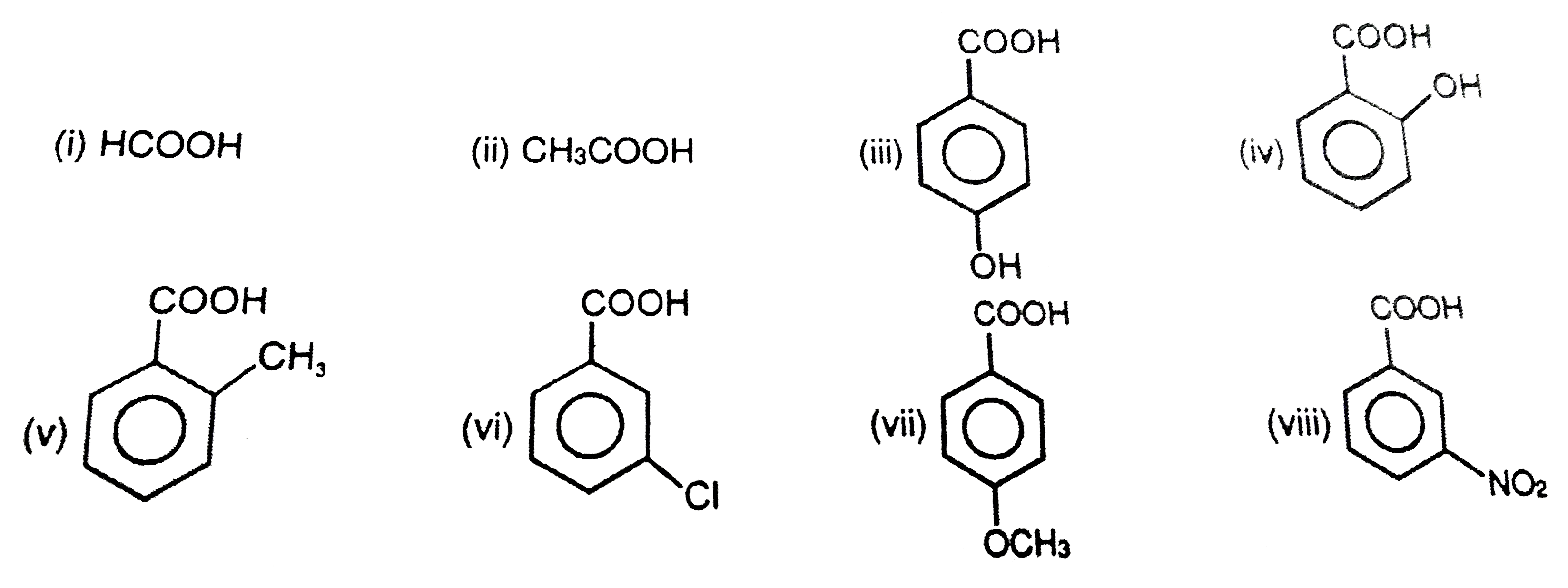
![PDF] Substituent effects on the electronic structure and pKa of benzoic acid | Semantic Scholar PDF] Substituent effects on the electronic structure and pKa of benzoic acid | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/7231c766a16c0fe0c564bc409846e5a74d693a18/2-TableI-1.png)