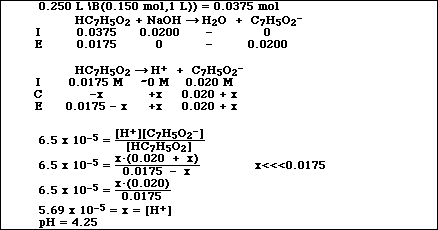Crystals | Free Full-Text | Co-Crystallization Kinetics of 2:1 Benzoic Acid–Sodium Benzoate Co-Crystal: The Effect of Templating Molecules in a Solution
![When a solution of benzoic acid was titrated with NaOH the pH of the solution when half the acid neutralized was 4.3 . Dissociation constant of the acid is [Use log 2 = 0.3] . When a solution of benzoic acid was titrated with NaOH the pH of the solution when half the acid neutralized was 4.3 . Dissociation constant of the acid is [Use log 2 = 0.3] .](https://haygot.s3.amazonaws.com/questions/1835171_829774_ans_40c76abc129644cfaff5cbb818338569.png)
When a solution of benzoic acid was titrated with NaOH the pH of the solution when half the acid neutralized was 4.3 . Dissociation constant of the acid is [Use log 2 = 0.3] .

Titration curves of benzoic acid for different analyte concentrations... | Download Scientific Diagram

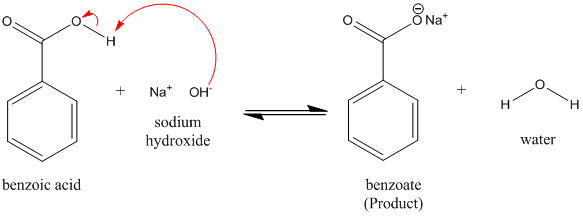
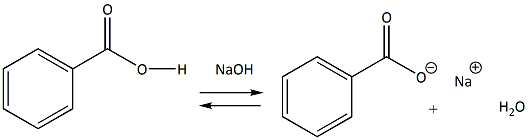
Draw the products of benzoic acid reacting with sodium hydroxide. Draw the products of the pyridine reacting with hydrochloric acid. Use the "+/-" button to add the charge (and H atom).

For the given reaction :2 phenylacetic acid + NaOH CaO , ⟶ ProductThe product is:A. Benzoic acidB. TolueneC. PhenolD. Benzene

SOLVED: A solution of benzoic acid (HC,HsOz) is titrated using sodium hydroxide: The Ka of benzoic acid is 6.4x 101. Write the balanced equation and the net ionic equation. 2.100.0 mL of

SOLVED: Sample Problem You titrate 100.0 mL ofa 0.025 M solution d benzoic acid with 0.100 M NaOH to the equivalencs point What is the pH when half of the benzoic acid

Write a balanced equation for the reaction of benzoic acid with hydroxide ion. Why is it necessary to extract the ether layer with sodium hydroxide? | Homework.Study.com

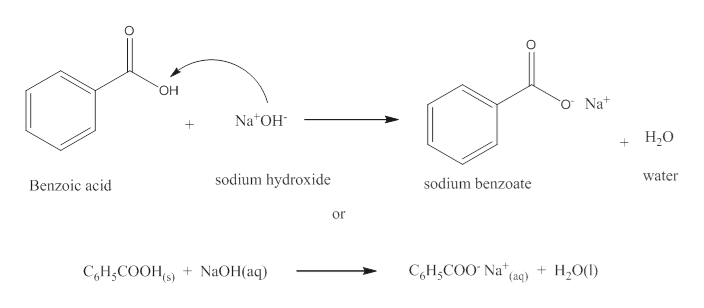
1. Draw a balanced chemical equation for the reaction that would occur between benzoic acid and aqueous sodium hydroxide. 2. Draw a balanced chemical equation for the reaction that would occur between
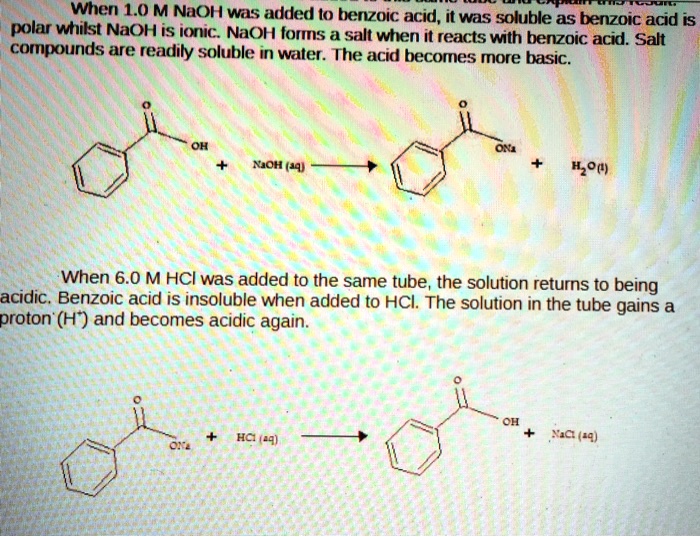
SOLVED: When 1.0 M NaOH was added (0 benzoic acid, it was soluble as benzoic acid is polar wtitst NaOH is ioric; NaOH forms salt when i reacts with berizoic aczo Sad
✓ Solved: Explain the results for the tube in which 1.0 M NaOH was added to benzoic acid. Write an equation...

Write the mechanism for the reaction of either benzoic acid or acetic acid with NaOH. Be sure to include all major structures and resonance forms. | Homework.Study.com

Write a chemical equation that explains the observations on the addition of HCL to the mixture of benzamide and NaOH subsequent to heating. | Homework.Study.com
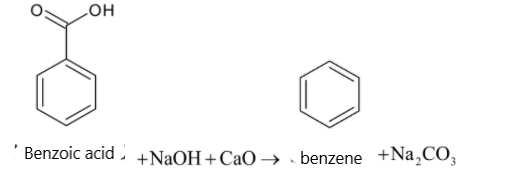
Heating of carboxylic acid with soda lime results in:A. dehydrationB. dehydrogenationC. decarboxylationD. addition of ${{{O}}_2}$