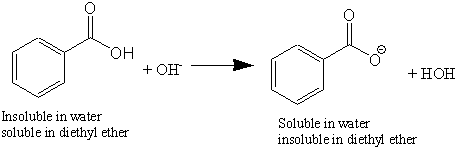
Physical chemical properties benzoic acid aromatic acids benzamide benzonitrile benzoyl chloride electrophilic substitution nitration halogenation reaction with alkalis carbonates benzonitrile benzamide advanced A level organic chemistry revision notes ...

57 mg dipentylamine is added to 122 mg benzoic acid and 2 mL water. Draw the structures of the species present in the water. | Homework.Study.com
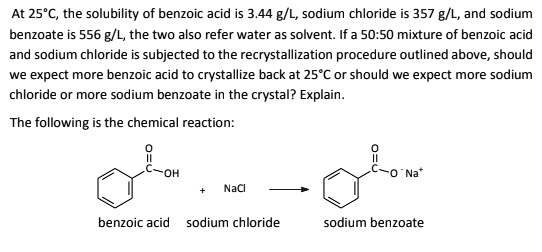
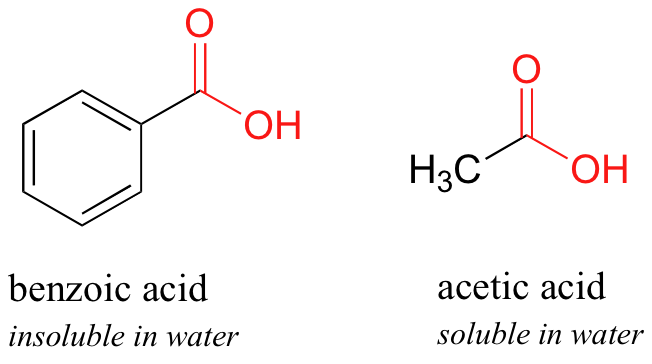
SOLVED: At 25*C, the solubility of benzoic acid is 3.44 g/L, sodium chloride is 357 g/L, and sodium benzoate 556 g/L, the two Iso refer water as solvent: If 50.50 mixture of

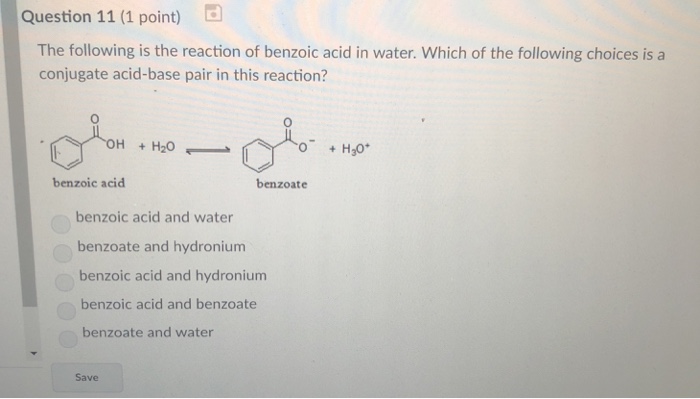
SOLVED: Write the equation for the dissociation of benzoic acid (CHsCOOH) in water. Cx HsCoo H F H Hz6 + C AsCoo b. Write the equilibrium equation for the reaction (Ka) C.

Solved) - Will sodium benzoate be more soluble in water than benzoic acid.... (1 Answer) | Transtutors

Temperature Dependent Solubility of Benzoic Acid in Aqueous Phase and Aqueous Mixtures of Aliphatic Alcohols

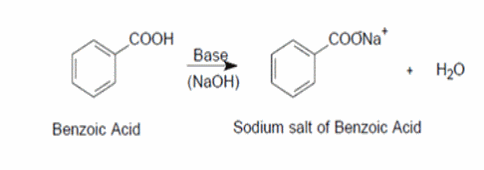
Explain why the following statement is incorrect: Benzoic acid dissolved in water upon the addition of a saturated solution of sodium bicarbonate. | Homework.Study.com