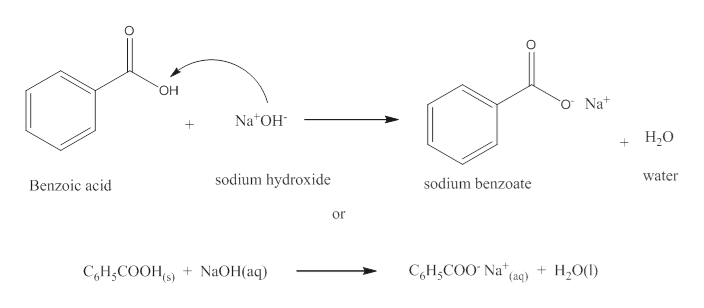
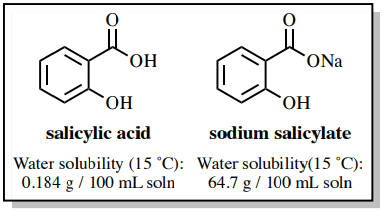
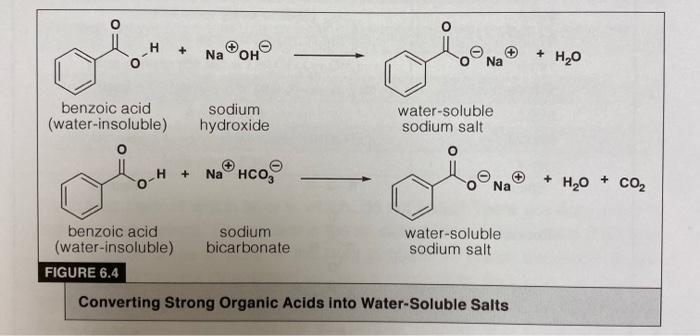
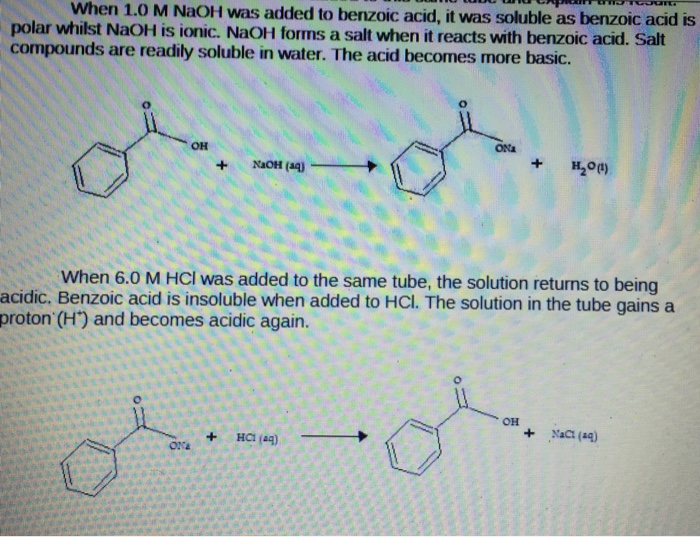
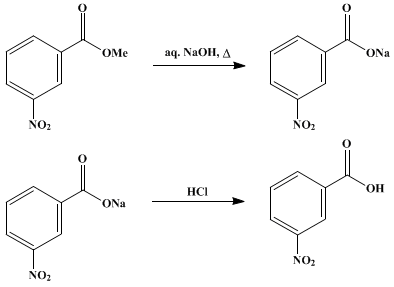
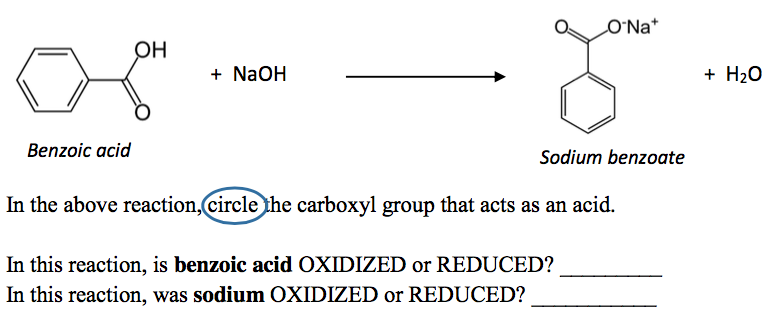Write a balanced equation for the reaction of benzoic acid with hydroxide ion. Why is it necessary to extract the ether layer with sodium hydroxide? | Homework.Study.com

Treatment of benzoic acid (C_6H_5CO_2H) with NaOH followed by 1-iodo-3-methylbutane forms H. H has a molecular ion at 192 and IR absorptions at 3064, 3035, 2960-2872, and 1721 cm^-1. Propose a structure

SOLVED: A solution of benzoic acid (HC,HsOz) is titrated using sodium hydroxide: The Ka of benzoic acid is 6.4x 101. Write the balanced equation and the net ionic equation. 2.100.0 mL of
Choose the correct representation of conductometric titration of benzoic acid vs sodium hydroxide. - Sarthaks eConnect | Largest Online Education Community
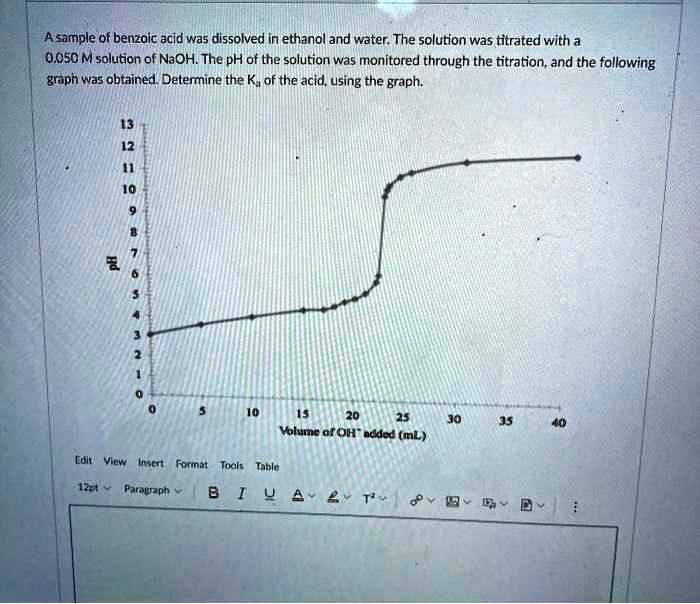
SOLVED: A sample of benzoic acid was dissolved in ethanol and water: The solution was titrated with a 0.O5O M solution of NaOH The pH of the solution was monitored through the
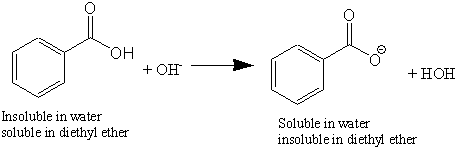
✓ Solved: When benzoic acid ( 5 ) is partitioned between diethyl ether and aqueous sodium hydroxide solution...

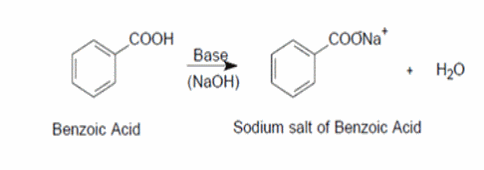
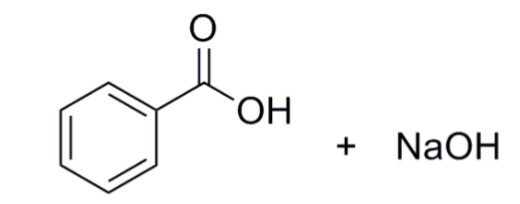
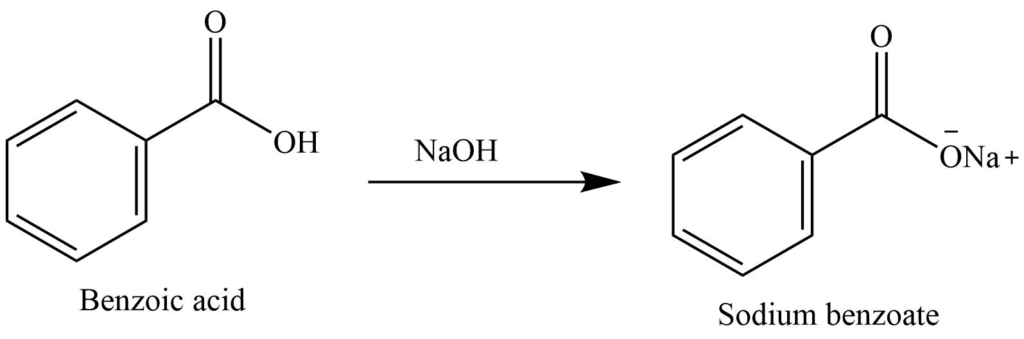
Draw a balanced reaction equation (using structures) for the reaction between benzoic acid and aqueous sodium hydroxide. | Homework.Study.com
![When a solution of benzoic acid was titrated with NaOH the pH of the solution when half the acid neutralized was 4.3 . Dissociation constant of the acid is [Use log 2 = 0.3] . When a solution of benzoic acid was titrated with NaOH the pH of the solution when half the acid neutralized was 4.3 . Dissociation constant of the acid is [Use log 2 = 0.3] .](https://haygot.s3.amazonaws.com/questions/1835171_829774_ans_40c76abc129644cfaff5cbb818338569.png)
When a solution of benzoic acid was titrated with NaOH the pH of the solution when half the acid neutralized was 4.3 . Dissociation constant of the acid is [Use log 2 = 0.3] .

Titration curves of benzoic acid for different analyte concentrations... | Download Scientific Diagram