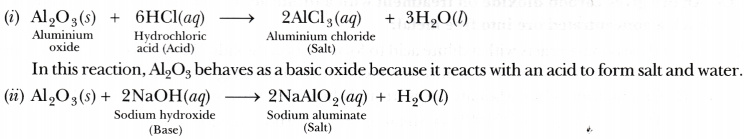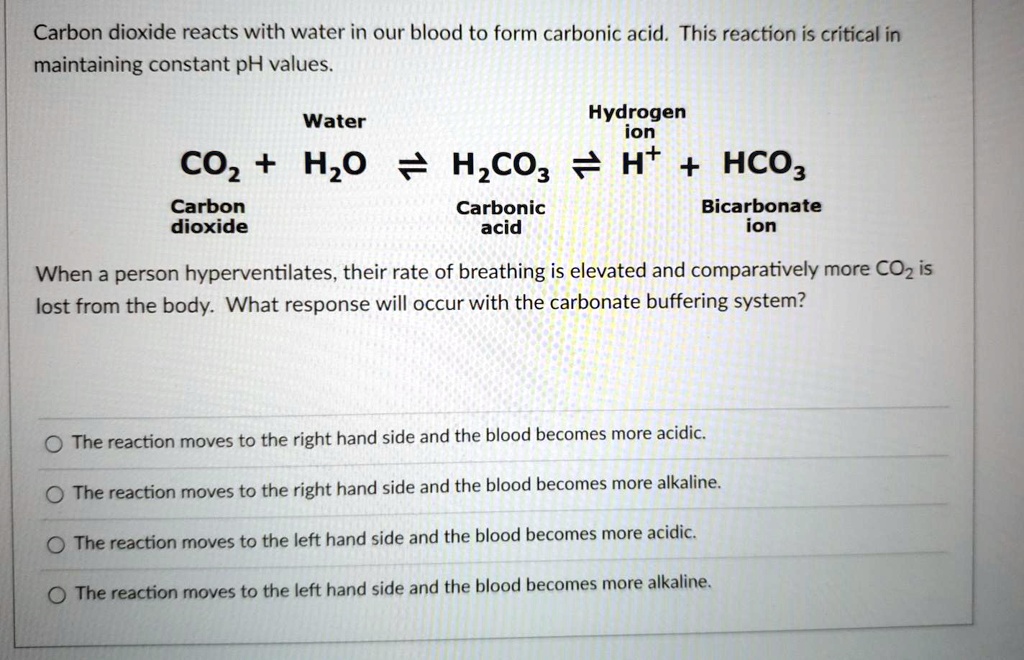
SOLVED: Carbon dioxide reacts with water in our blood to form carbonic acid. This reaction is critical in maintaining constant pH values Water Hydrogen ion HzCOz H+ HCO3 Carbonic Bicarbonate acid ion

Title: Lesson 4 Period 3 Oxides Learning Objectives: Understand and explain the trend in acid-base behaviour of the period 3 oxides Complete an experiment. - ppt download
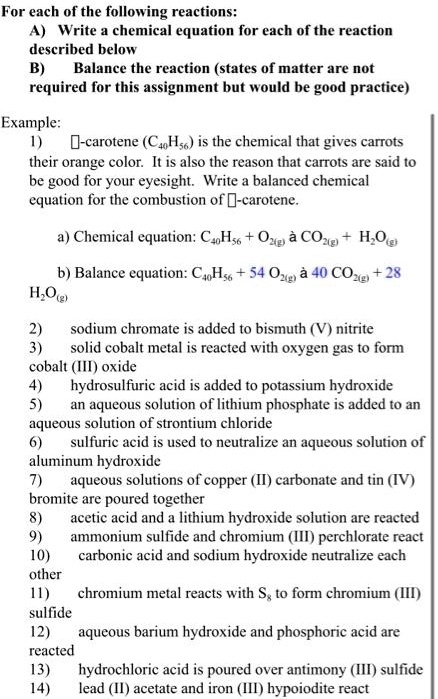
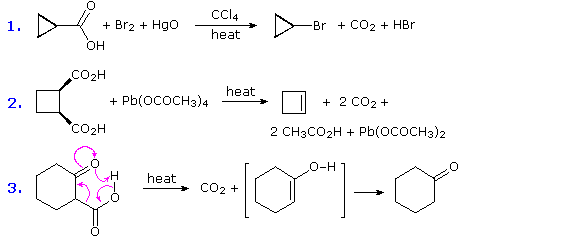
SOLVED: For each of the following reactions: Write chemical equation for each of the reaction described below Balance the reaction (states of matter are not required for this assignment but would be
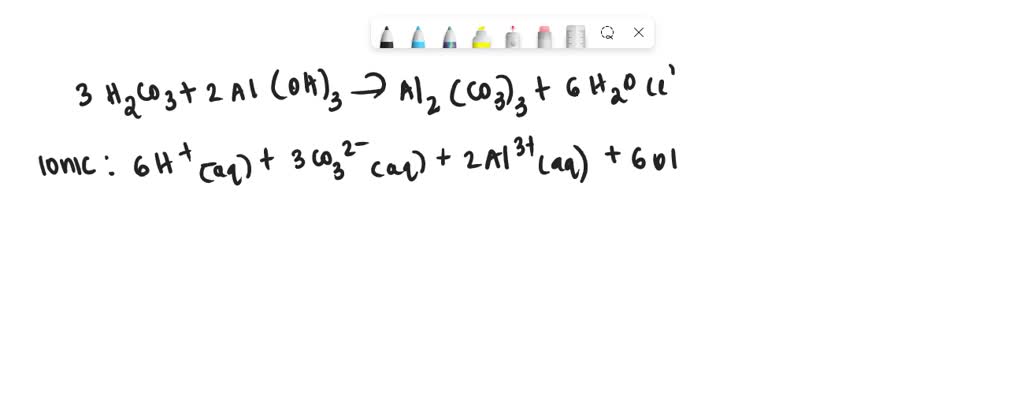
SOLVED: When solutions of carbonic acid and aluminum hydroxide react, which of the following are NOT present in the net ionic equation? I. hydrogen ion II. carbonate ion III. aluminum ion IV.
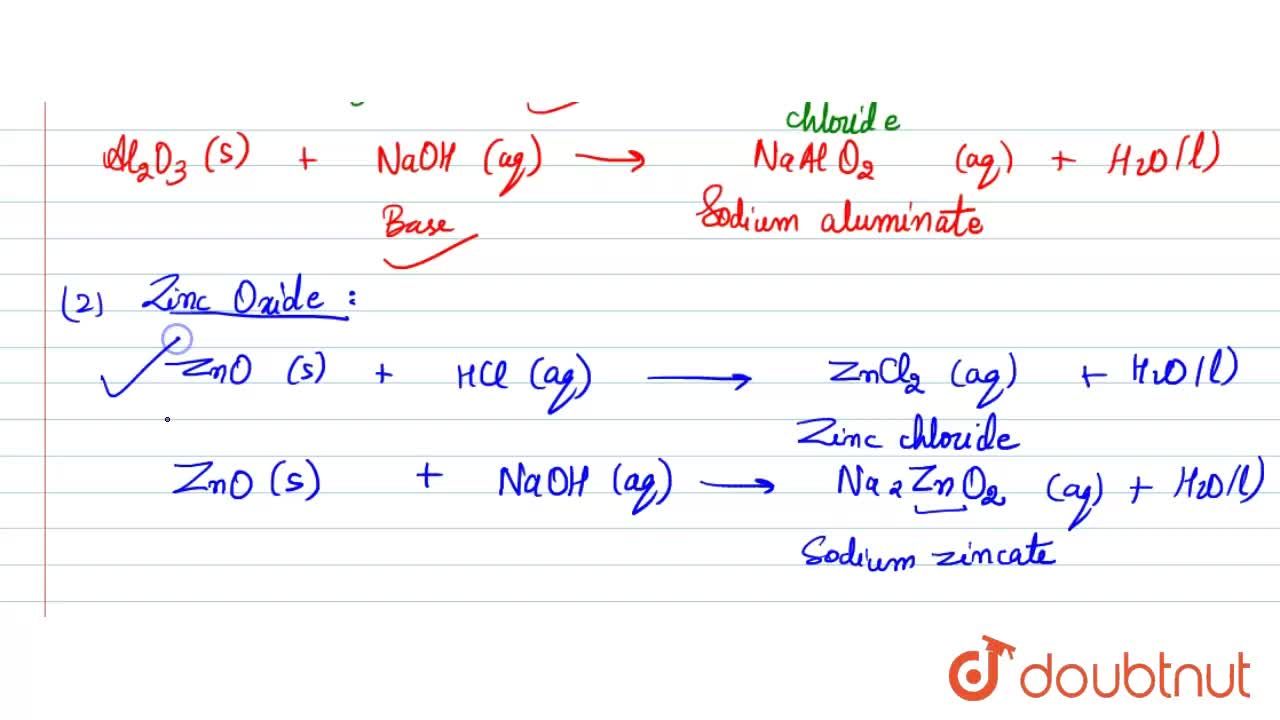
Aluminium oxide and zinc oxide react with both acids are bases to produce salt and water. What are these oxides called ? Write chemical equation in each case.

Write balanced equations by predicting the products of the following reactions. Include the physical state of each element or compound. A single-replacement reaction of calcium metal with a nitric acid solution.

SOLVED: QuESTiON points Save Answe 0 g of aluminum hydroxide reacts with 25.0 mL of 0.500 M carbonic acid according t0 the following unbalanced reaction. AI(OH)3(s) H2CO3(aq) Al2(CO3)3(s) HzO() The mass of