
d Write the balanced chemical equations for the following reaction: Aluminium reacts with dilute hydrochloric acid e Name - Chemistry - Materials Metals and Non-metals - 15866735 | Meritnation.com
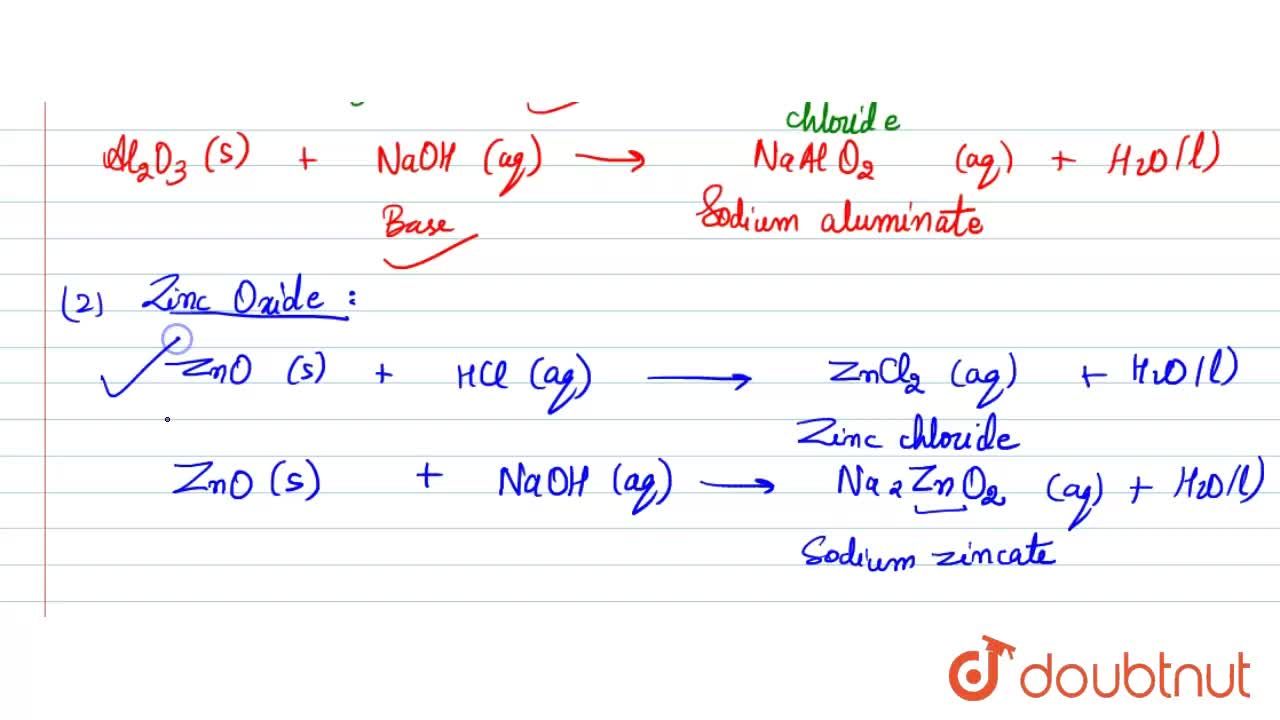
Aluminium oxide and zinc oxide react with both acids are bases to produce salt and water. What are these oxides called ? Write chemical equation in each case.
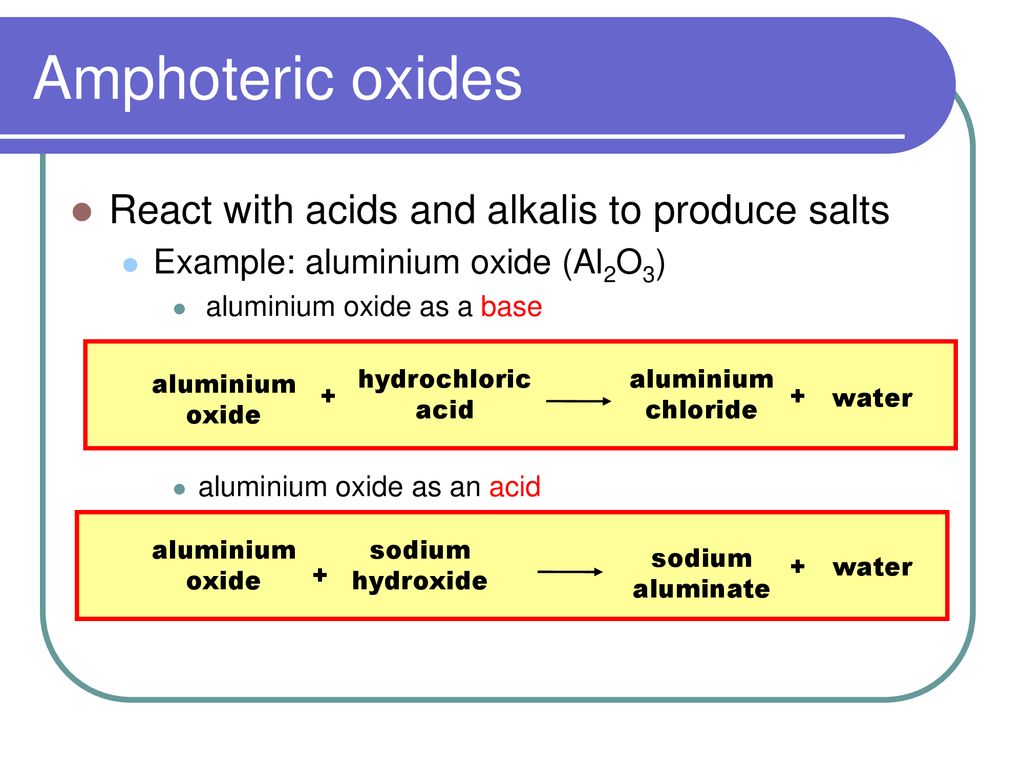
What is an oxide? An oxide is a Binary compound of oxygen and another element. M & O Oxides can be classified in two ways – Nature of Oxides Amount of. -

Al2O3+HCl=AlCl3+H2O Balanced Equation||Aluminium oxide + Hydrochloric acid Balanced Equation - YouTube

What do you call the metal oxide having both acidic and basic properties. Give two examples. Give - Brainly.in















