Chapter 17 Acid-Base & Solubility Equilibria The Common Ion Effect 17.2 Buffer Solutions 17.3Acid-Base Titrations (omitted) 17.4Solubility Equilibria. - ppt download
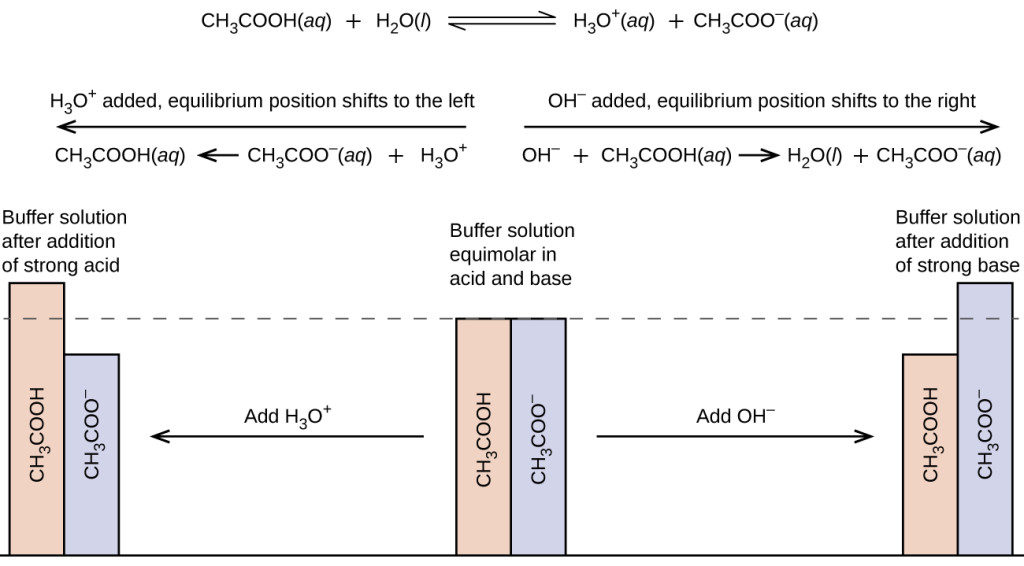
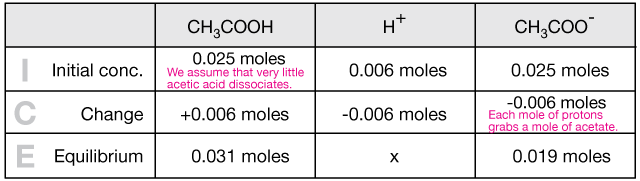
When a small amount of HCL is added to a buffer solution of acetic acid and sodium acetate what happen?
How to prepare 1oo ml of 0.200 M acetate buffer at pH 5.00 starting with pure liquid acetic acid and solutions containing 3M HCl and 3M NaOH - Quora

SOLVED: Question 12 (1 point) What reaction occurs as HCl solution is added to a buffer solution containing equal concentration of acetic acid, CH3COOH; and sodium acetate, CH3COONa? CHzcoo CHzCOoH CHzCOOH Ht

Titration of CH3COONa with HCl and pKa determination from half equivalence point - Chemistry Stack Exchange
![Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ] Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]](https://i.ytimg.com/vi/t9B5VgPOTG4/maxresdefault.jpg)
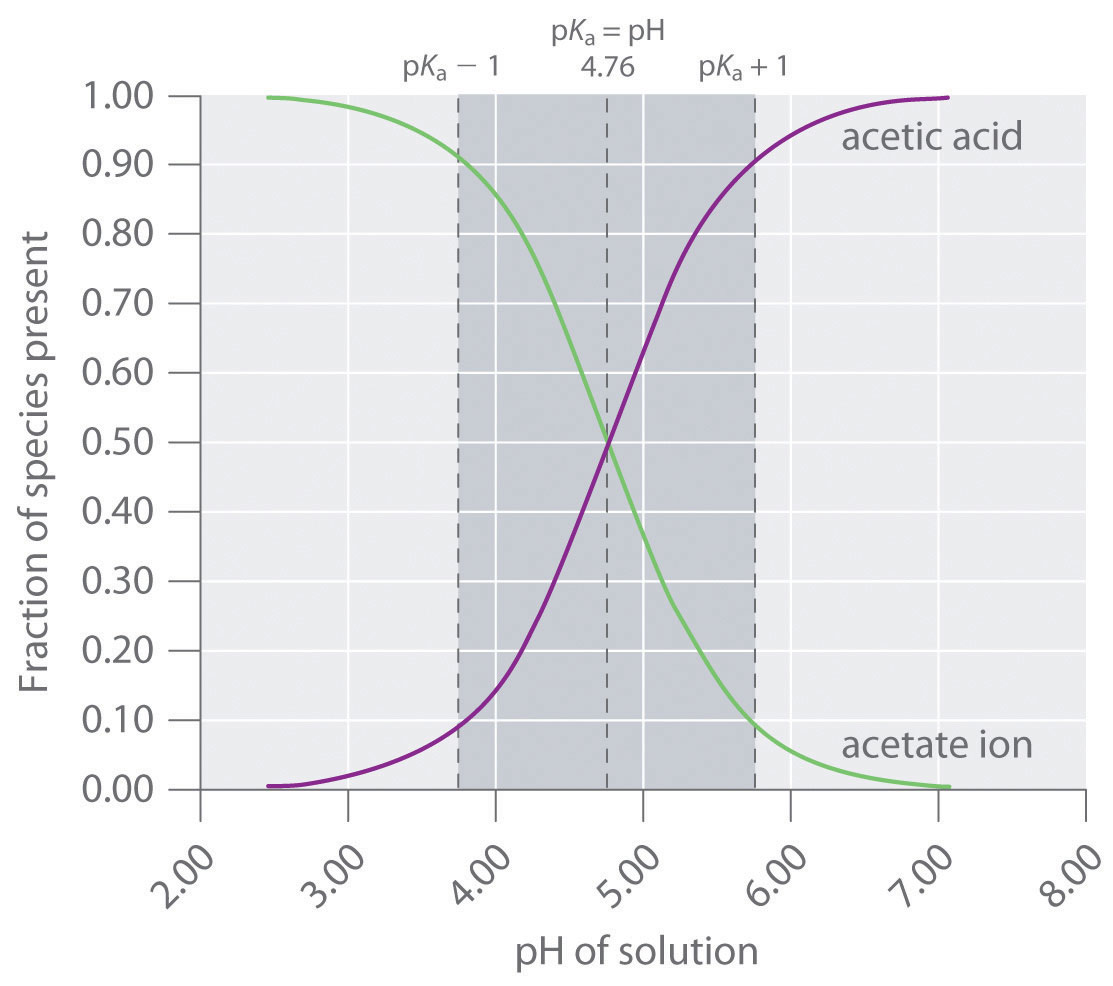
Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]

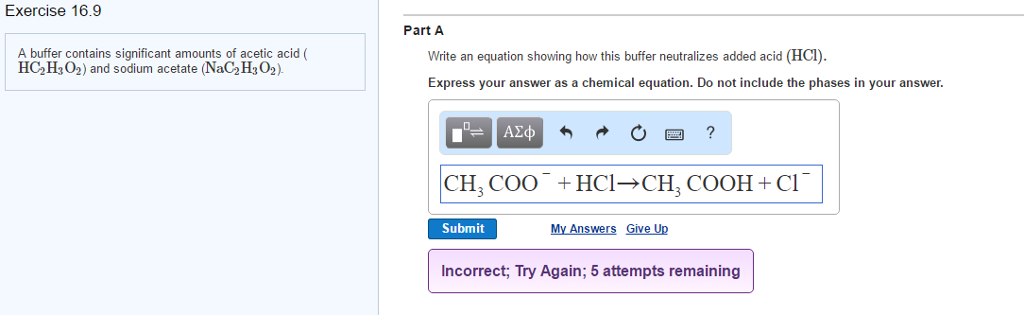
OneClass: A buffer contains significant amounts of acetic acid and sodium acetate. Write an equation ...

You have 250mL of a 0.56M solution of sodium acetate. How many mL of 0.50M acetic acid should be added to make a buffer of pH 4.40? | Homework.Study.com