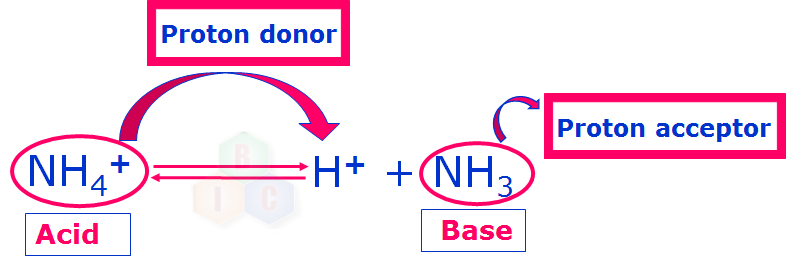
Proton Donors & Acceptors (6/10) | Chemical Reactivity - NCEA Level 2 Chemistry | StudyTime NZ - YouTube

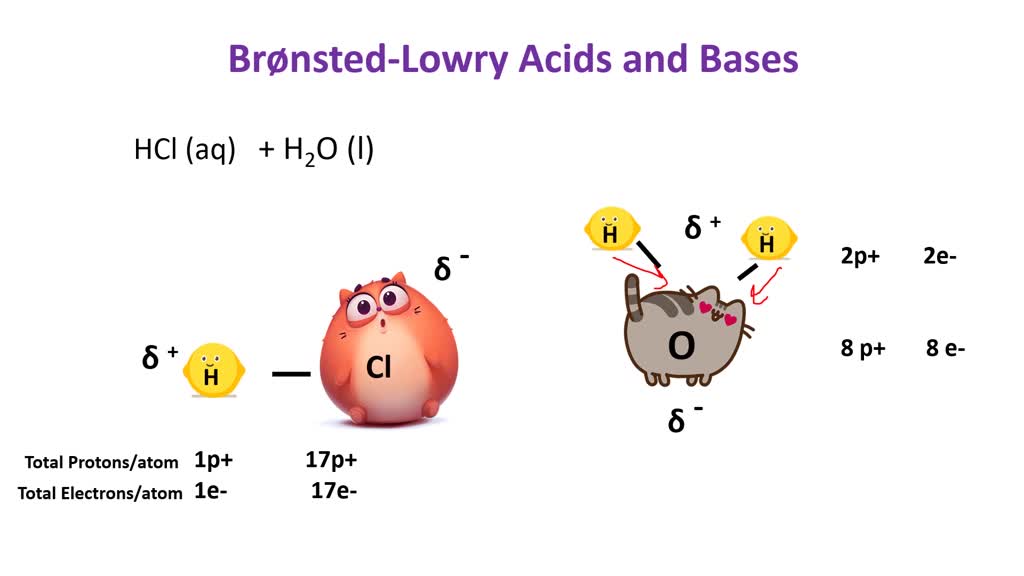
16/10/2015 Acids and Bases Hydrochloric acidCitric acidWater An acid is a “proton donor”: A base is a “proton acceptor”: H Cl H +- H O Na H O - + Sodium. - ppt download

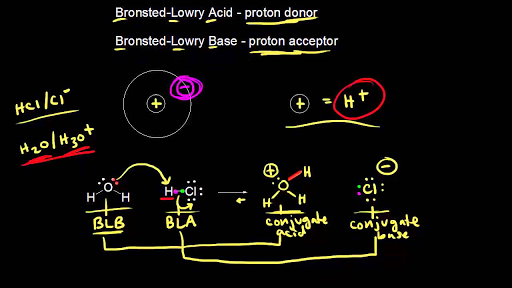
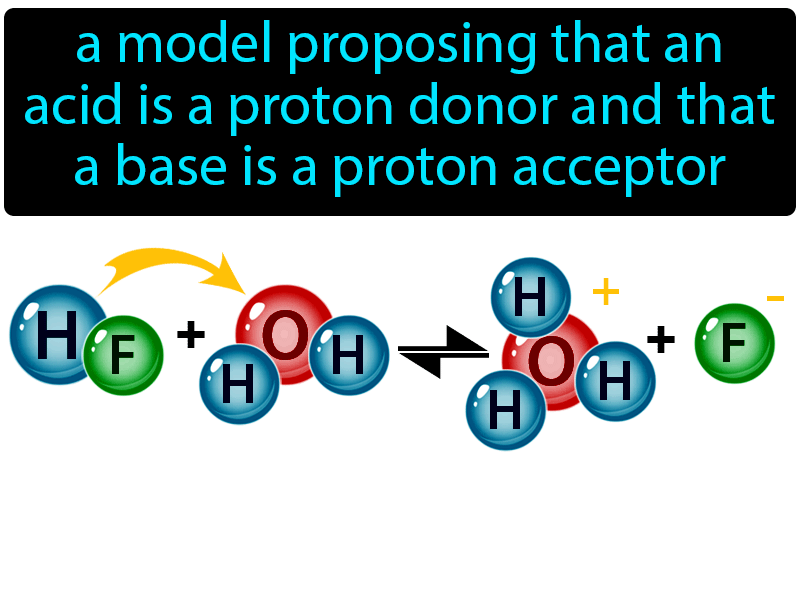
Acids and Bases. Acids & Bases The Bronsted-Lowry model defines an acid as a proton donor. A base is a proton acceptor. Note that this definition is based. - ppt download
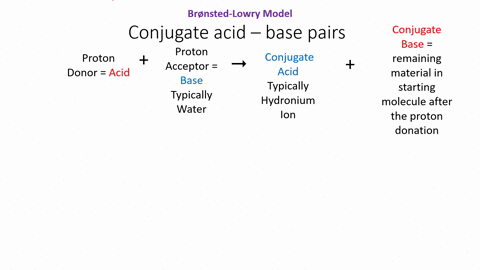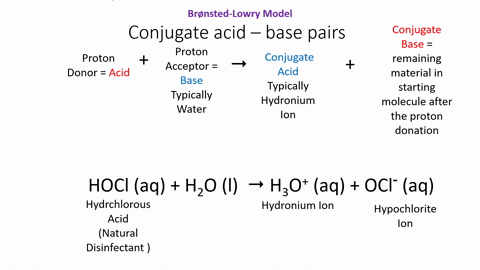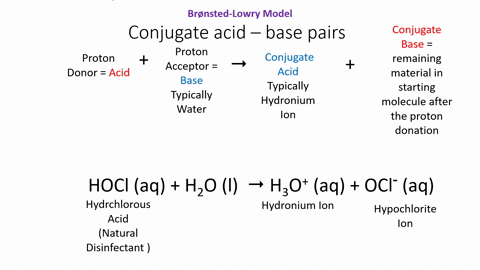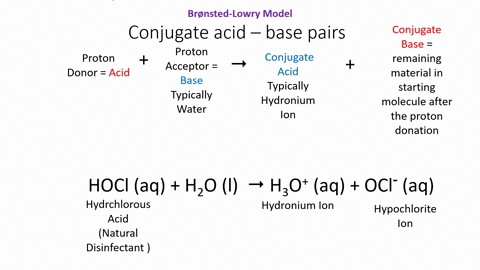
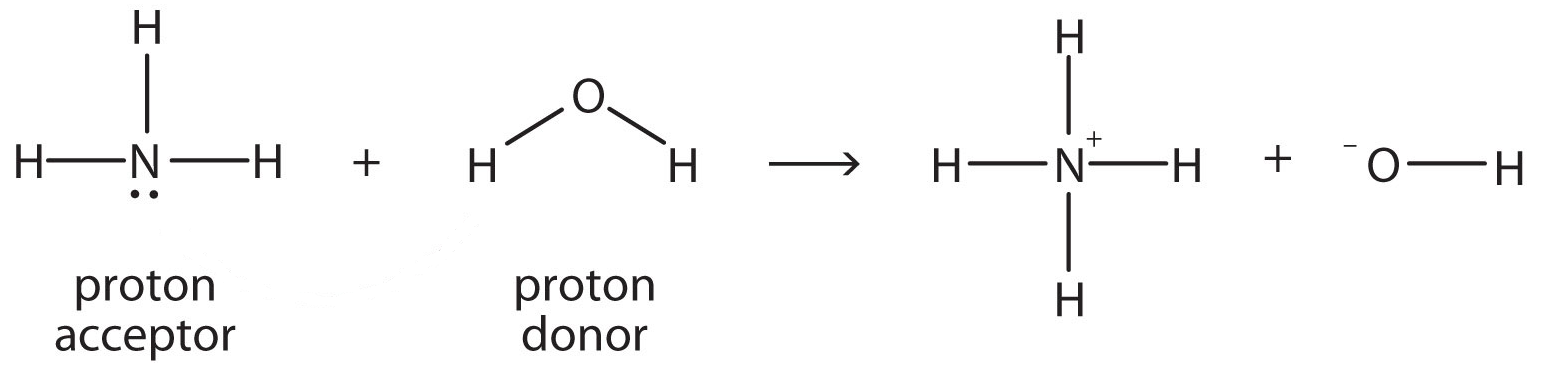


.PNG)

/chapter3/pages19and20/page19and20_files/abexample.png)

/chapter3/pages19and20/page19and20_files/lewisbronsted.png)