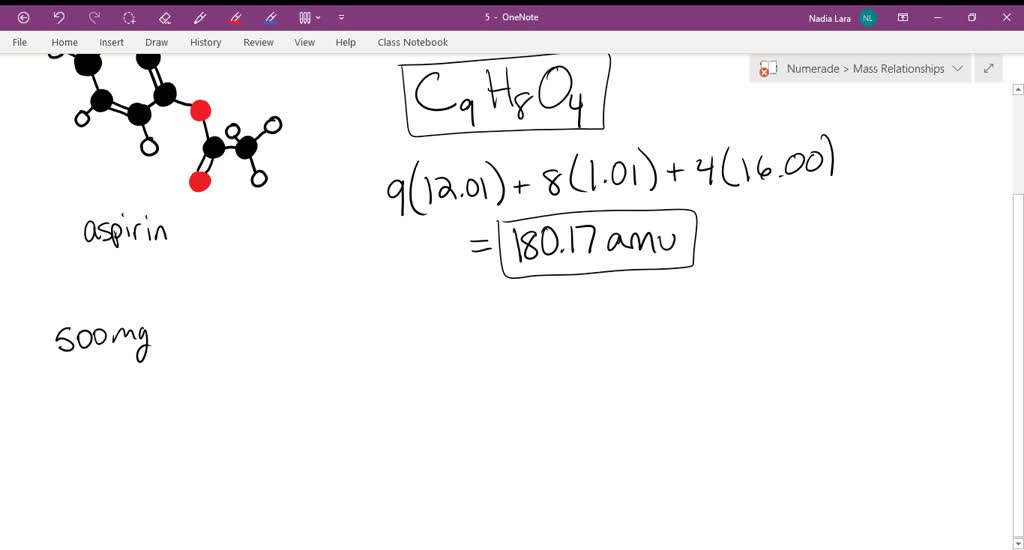
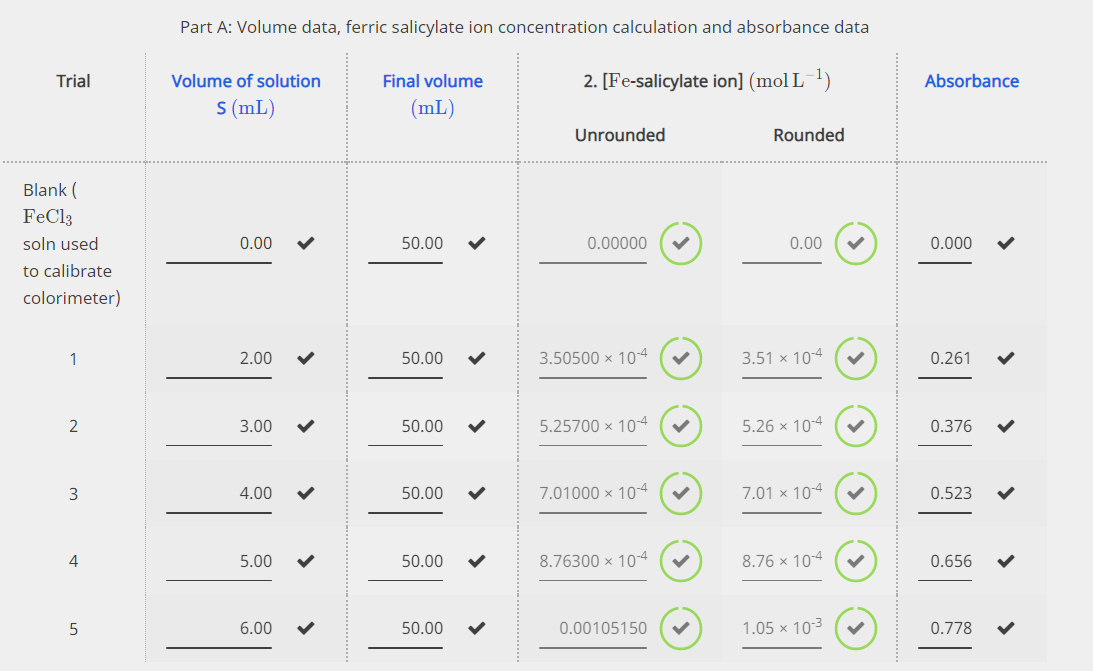
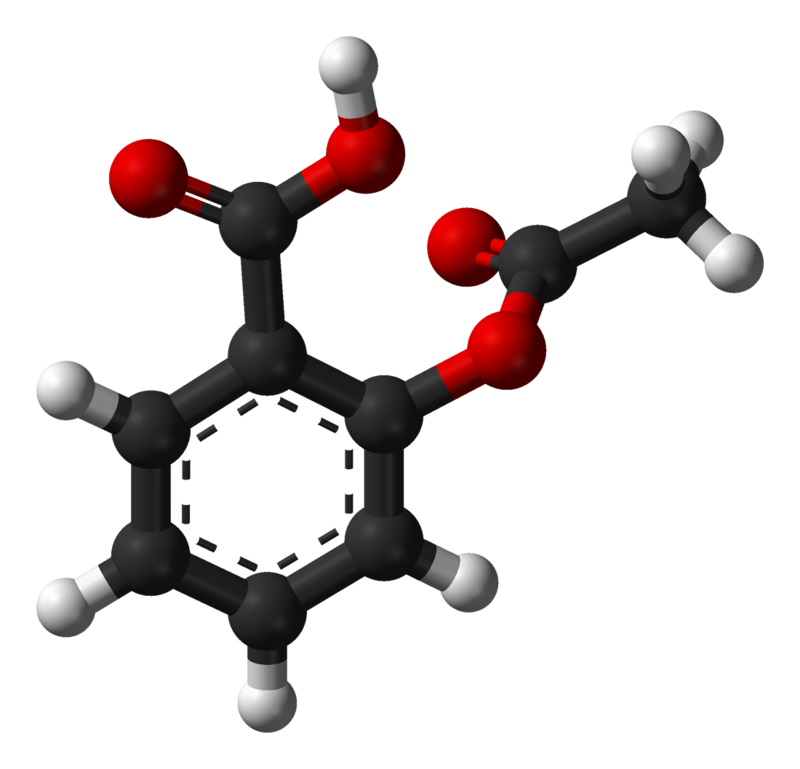
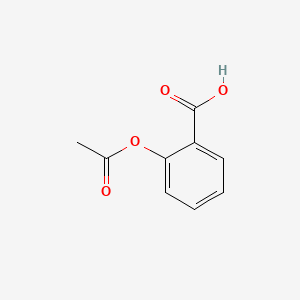
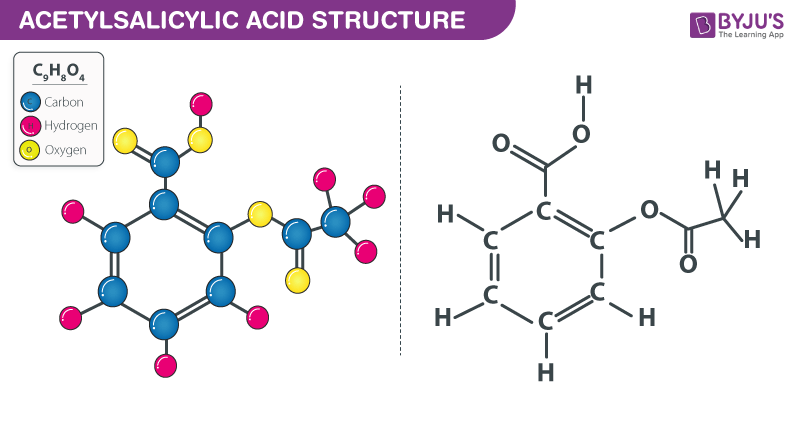
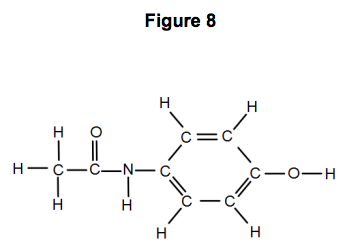
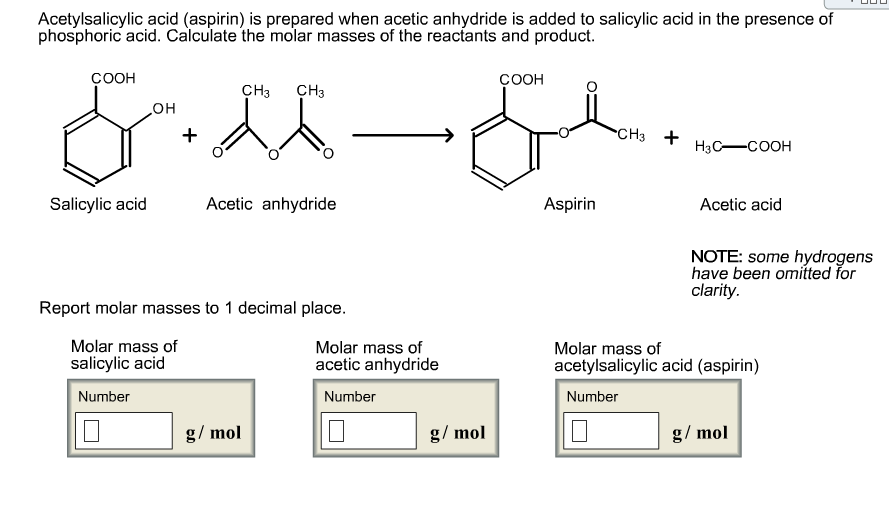
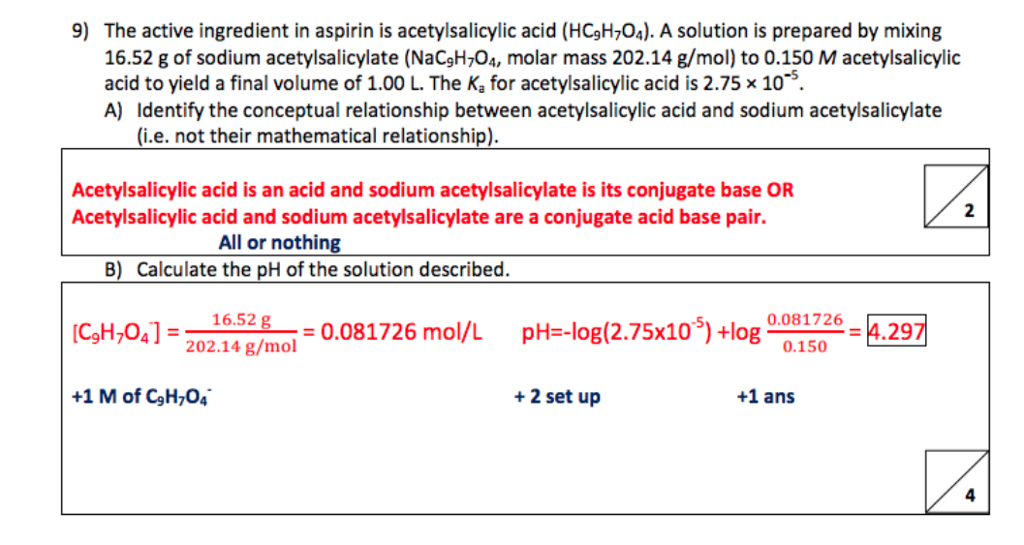
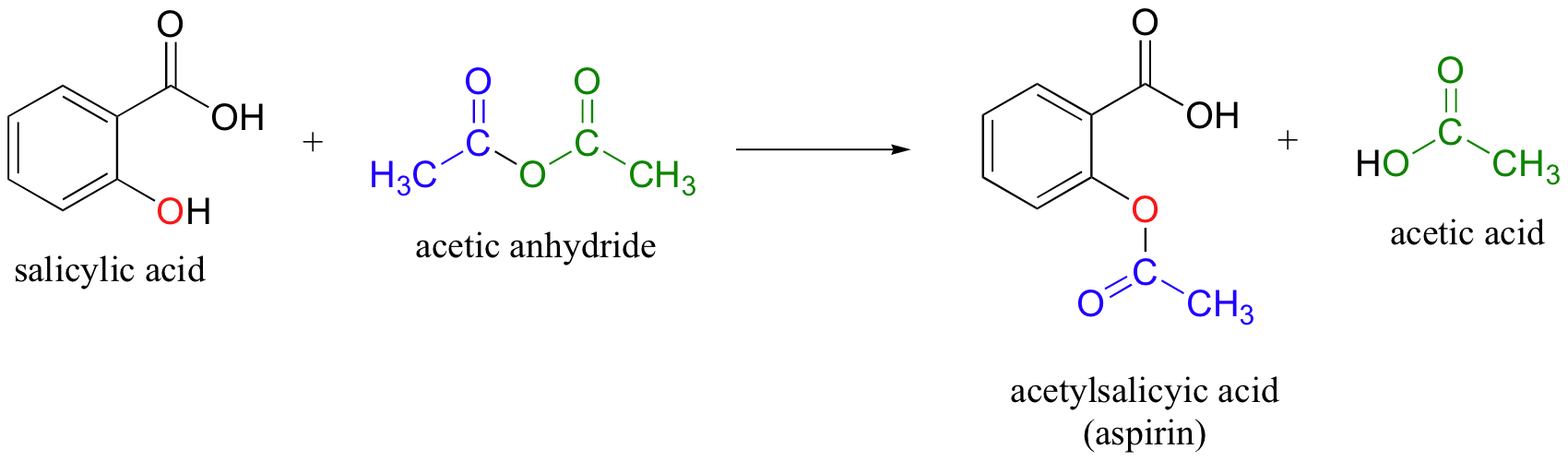
Sketch the molecular structure of acetylsalicylic acid and calculate its molar mass. | Homework.Study.com

SOLVED: The molar mass of salicylic acid (CzH6O3) is 138 g/mole: The molar mass of [acetylsalicylic acid (CgH,O4) is 180 g/mole. Calculate the theoretical yield of acetylsalicylic acid (aspirin) based on thc

SOLVED: (T) Aspirin (CoHsO+: molar mass = 180.157 glmol) is produced from the reaction of salicylic acid (C-HsOa, molar mass = 138.1207 g/mol) and acetic anhydride (CAHsO3 molar mass 102.0887 g/mol): CzHsO3 +
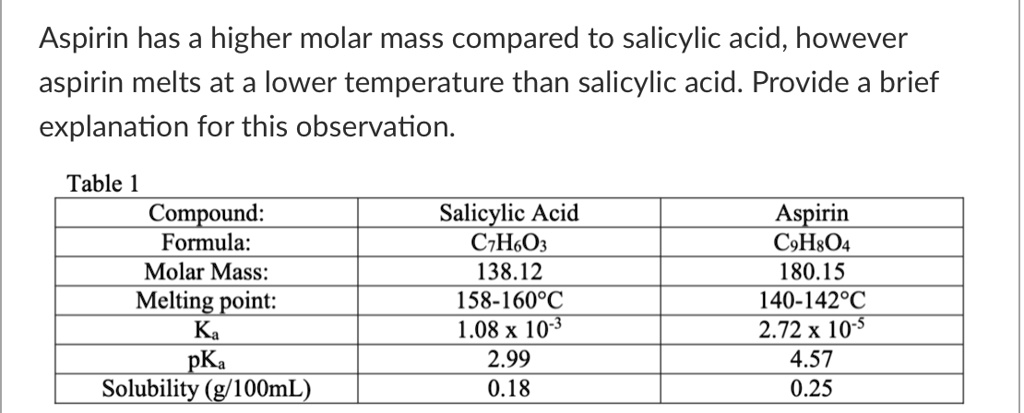
SOLVED: Aspirin has a higher molar mass compared to salicylic acid, however aspirin melts at a lower temperature than salicylic acid. Provide a brief explanation for this observation: Table 1 Compound: Formula: