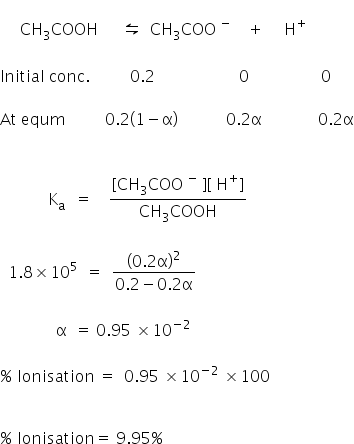
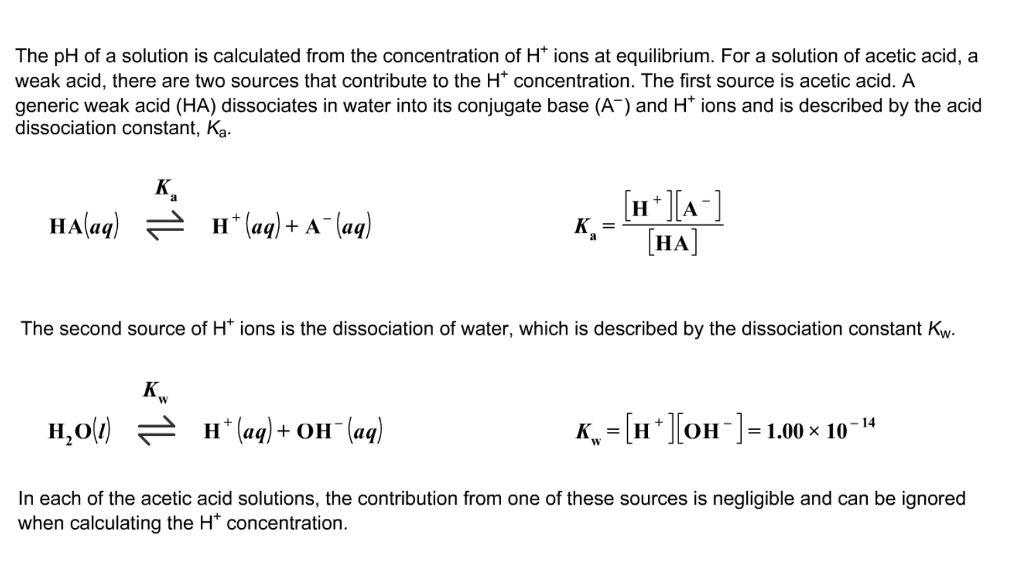
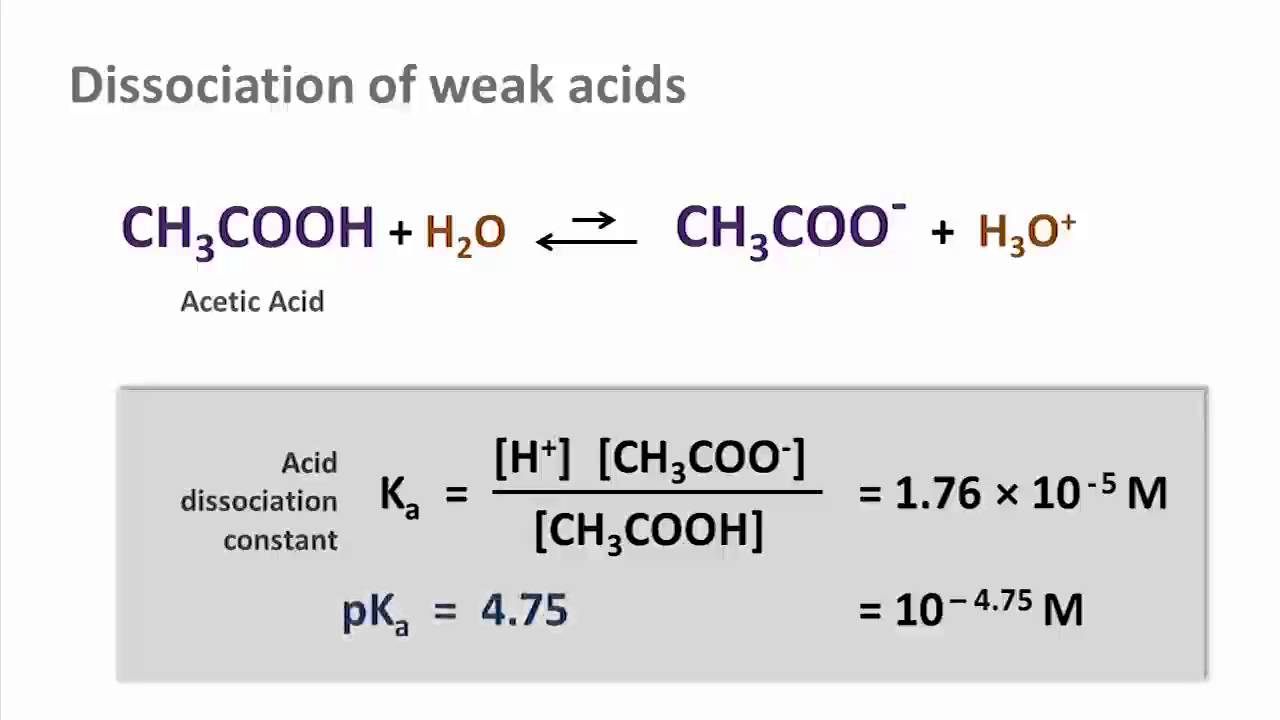
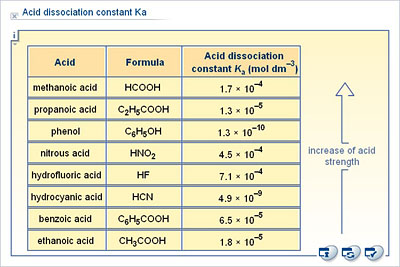
The degree of dissociation of acetic acid in a 0.1 M solution is 1.32 × 10^–2. Calculate dissociation constant of acid and its pKa value : - Sarthaks eConnect | Largest Online Education Community

The ionization constant of acetic acid `1.74xx10^(-5)`. Calculate the degree of dissociation - YouTube

The ionization constant of acetic acid `1.74xx10^(-5)`. Calculate the degree of dissociation of ... - YouTube

iv. Dissociation constant of aceticacid is 1.8 x 10-5. Calculate percentdissociation of acetic acid in 0.01 - Brainly.in

Value of dissociation constant of acetic acid is 10^-6 , where as dissociation constant of formic acid is 10^-5 . Which of the following will be the value of pKa (acetic acid) - pKa (formic acid)?
The dissociation constant for acetic acid and HCN at 25°C are 1.5 x 10^-5 and 4.5 x 10^-10 respectively. the equilibrium constant - Sarthaks eConnect | Largest Online Education Community

The dissociation constant of acetic acid at a given temperature is 1.69 × 10^-5 . The degree of dissociation of 0.01 M acetic acid in the presence of 0.01 M HCI is equal to:

The dissociation constants for acetic acid and HCN at 25C are 1.5 x 10^-5 and 4.5 x 10^-10 respectively. The equilibrium constant for the equilibrium CN + CH3COOH HCN + CH3COO - would be ?

PDF) Dissociation Constant of Acetic Acid in (N,N-Dimethylformamide + Water) Mixtures at the Temperature 298.15 K

The dissociation constant of acetic acid at a given temperature is 1.69xx10^(-5).The degree of dissociation of 0.01 M acetic acid in presence of 0.01 M HCl is equal to :

The dissociation constant of acetic acid at a given temperature is 1.69 × 10^-5 . The degree of dissociation of 0.01 M acetic acid in the presence of 0.01 M HCl is equal to:

Find dissociation constant for acetic acid when 0 6 g of it is dissolved in 100 g of water with - Chemistry - Solutions - 12760283 | Meritnation.com

The ionization constant of acetic acid is 1.74 × 10 5 . Calculate the degree of dissociation of acetic acid in its 0.05 M solution. Calculate the concentration of acetate ion in the solution and its pH.