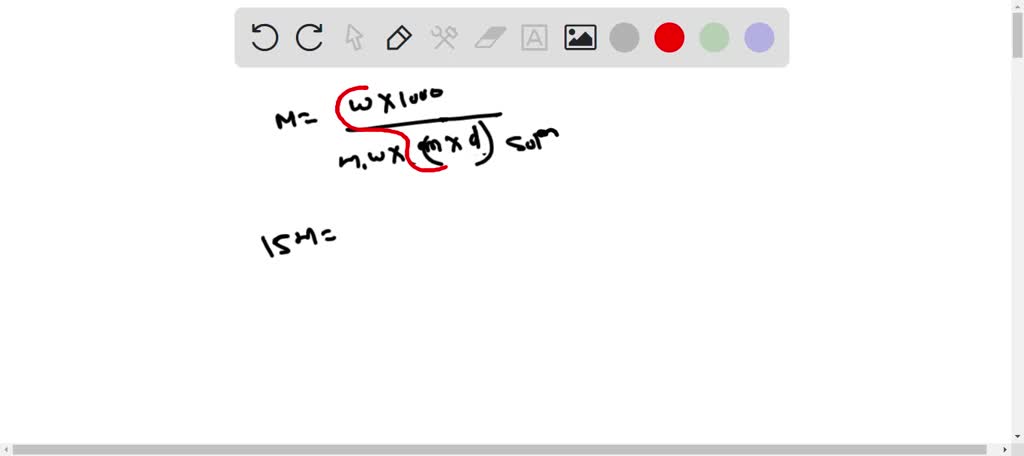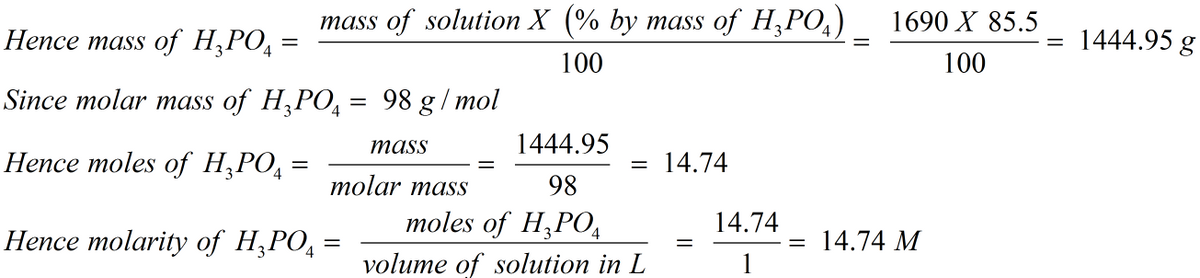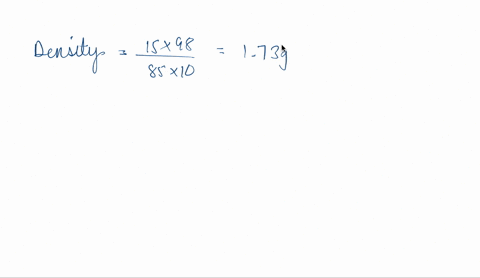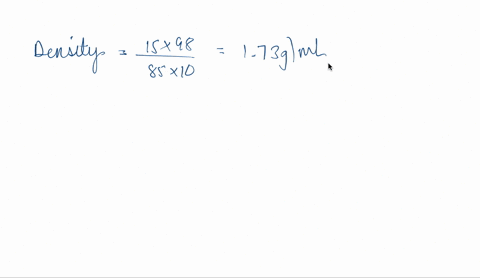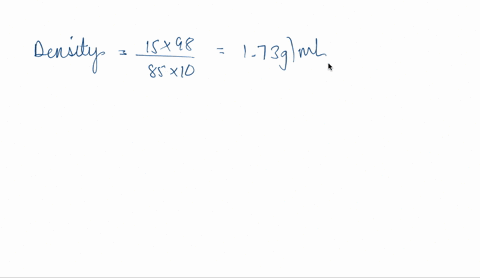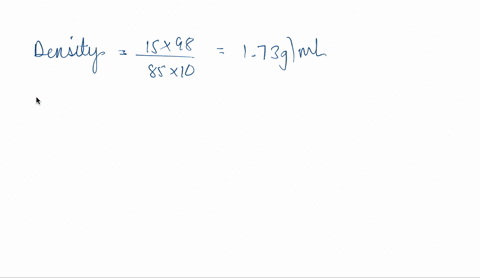
SOLVED: phosphoric acid, 85% purity density =1.88 g/ml) AND the Calculate the volume of commercial of water needed to prepare SOOmL of 0.5 M phosphoric acid solution. (MW: 98g/mol) volume (1 pt)
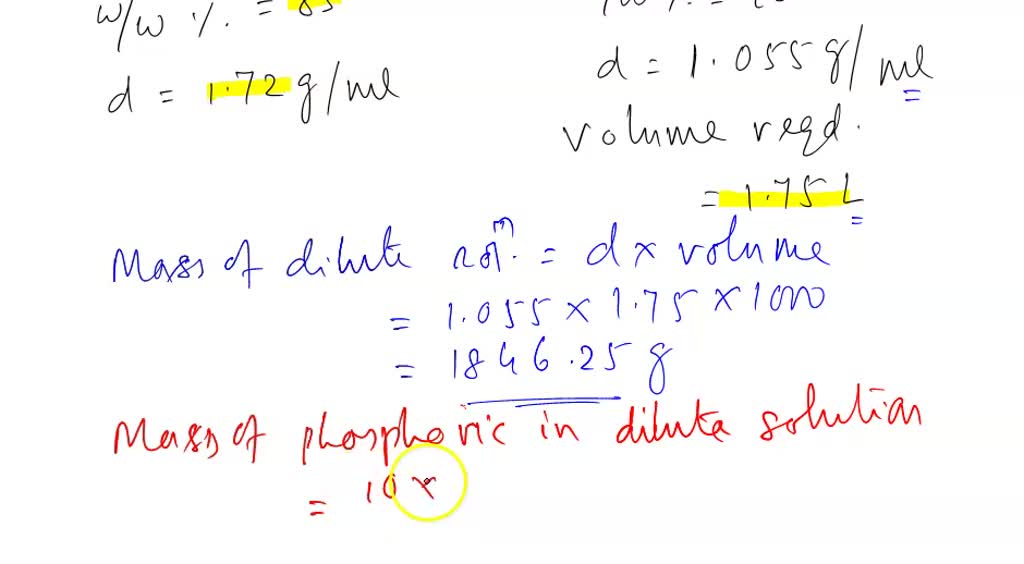
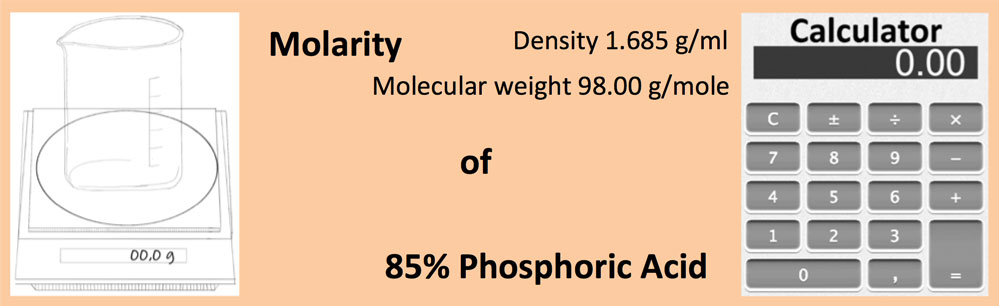
SOLVED: an 85% (w/w%) solution of phosphoric acid has a specific gravity of 1.6845 g/ml. (a)Find the normality of the phosphoric acid solution. (b) How many mmoles of the acid are present

Influence of phosphoric acid concentration in the mixture on product pH. | Download Scientific Diagram

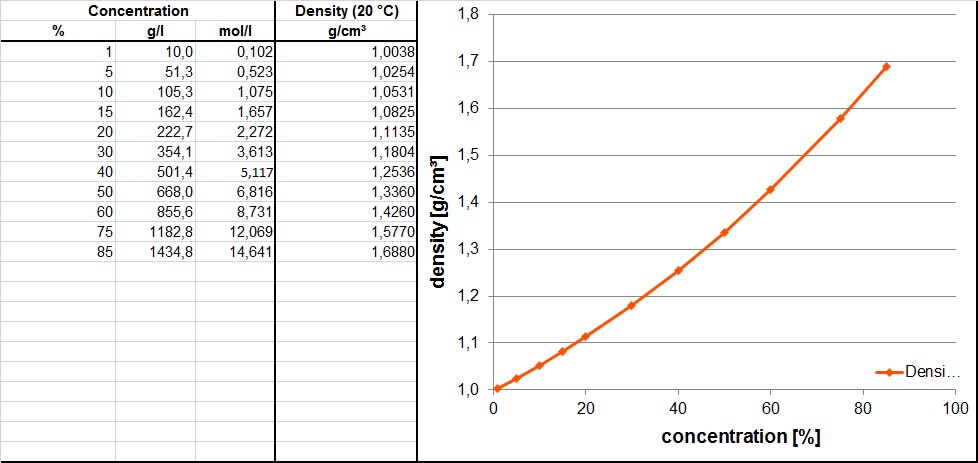
A concentrated phosphoric acid solution is 85.doc - A concentrated phosphoric acid solution is 85.5% H3PO4 by mass and has a density of 1.69 g/mL at | Course Hero

Solved) - A concentrated phosphoric acid solution is 85.5% H3PO4 by mass and... (1 Answer) | Transtutors

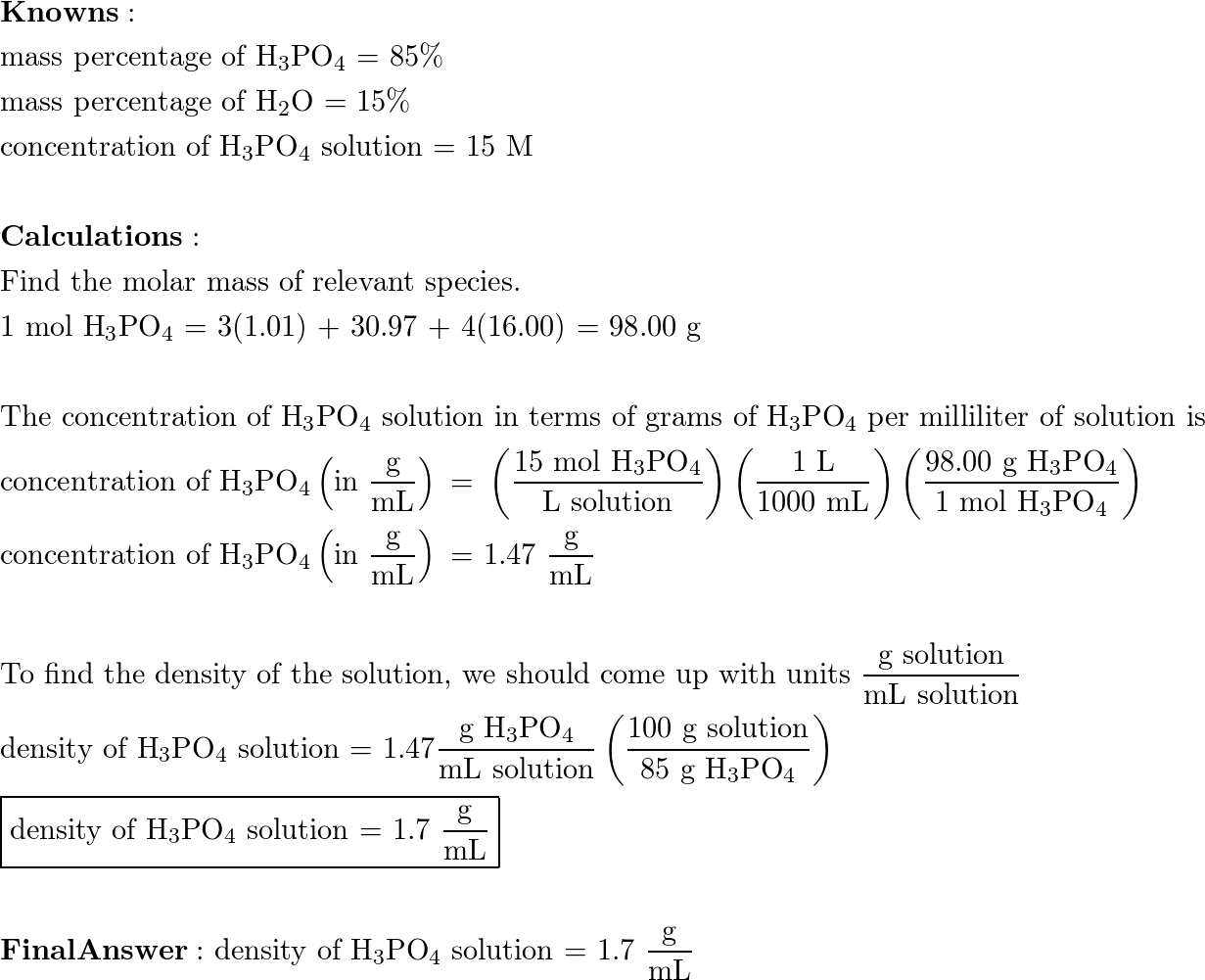
SOLVED: A concentrated phosphoric acid solution is 85.5% H3PO4 by mass and has a density of 1.69 g/mL at 25°C. What is the molarity of H3PO4? What is the mole fraction of

SOLVED: A solution of phosphoric acid was made by dissolving 10.1 g of H3PO4 in 136.00 mL of water. The resulting volume was 140. mL. Calculate the density, mole fraction, molarity, and

Selection of stainless steels for handling phosphoric acid (H3PO4) – British Stainless Steel Association